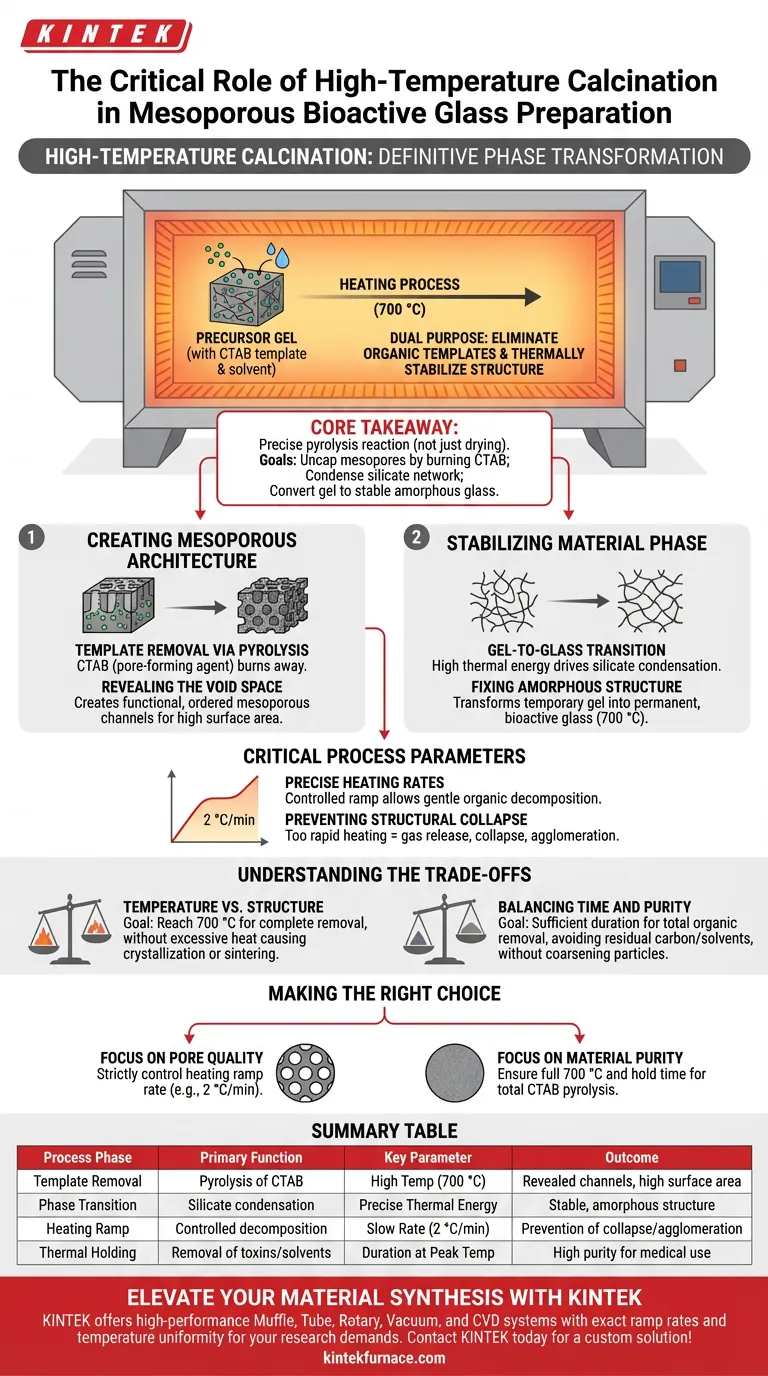
High-temperature calcination is the definitive phase transformation step in the synthesis of mesoporous bioactive glass particles. It serves the dual purpose of eliminating organic templates to reveal the porous architecture and thermally stabilizing the material's chemical structure.
Core Takeaway Calcination in a muffle furnace (typically at 700 °C) is not merely a drying process; it is a precise pyrolysis reaction. Its primary goal is to burn off the CTAB pore-forming agent to "uncap" the mesopores while simultaneously condensing the silicate network to convert the precursor gel into a stable, bioactive amorphous glass.
Creating the Mesoporous Architecture
Template Removal via Pyrolysis
The central role of the muffle furnace is to facilitate the high-temperature pyrolysis of CTAB (cetyltrimethylammonium bromide). During the initial synthesis, CTAB acts as a "pore-forming agent" or template around which the glass structure forms.
Revealing the Void Space
Once the structure is built, the CTAB must be removed to create the functional porosity. Calcination burns this organic template away completely. The removal of the template is what physically creates the ordered mesoporous channels within the nanoparticles, which are critical for the material's surface area and reactivity.
Stabilizing the Material Phase
Gel-to-Glass Transition
Before calcination, the material exists in a "gel state." The high thermal energy provided by the furnace drives the condensation of the silicate network.
Fixing the Amorphous Structure
At 700 °C, the process stabilizes this network, effectively transforming the temporary gel into a permanent, bioactive amorphous glass phase. This structural fixation is essential for the material's mechanical stability and its ability to function in biological environments.
Critical Process Parameters
Precise Heating Rates
According to supplementary data on structural integrity, the rate at which the muffle furnace heats is just as important as the final temperature. A controlled rate (often 2 °C/min) is critical.
Preventing Structural Collapse
A slow, controlled ramp allows for the gentle decomposition of organic molecules. If the heating is too rapid, the sudden release of large volumes of gas from the decomposing CTAB can cause the nanoparticle structure to collapse or lead to severe agglomeration, destroying the desired pore size distribution.
Understanding the Trade-offs
Temperature vs. Structure
While high temperatures are required to remove the CTAB, excessive thermal aggression can be detrimental. The goal is to reach the target temperature (700 °C) to ensure complete removal of organics without inducing unwanted crystallization or sintering that could reduce surface area.
Balancing Time and Purity
The process requires sufficient duration to ensure no residual organic surfactants remain. Incomplete calcination leaves behind carbon residues or toxic solvents, rendering the bioactive glass unsuitable for medical applications. However, extending the time unnecessarily wastes energy and risks coarsening the particles.
Making the Right Choice for Your Goal
- If your primary focus is Pore Quality: Strictly control the heating ramp rate (e.g., 2 °C/min) to prevent gas-induced structural collapse during template decomposition.
- If your primary focus is Material Purity: Ensure the furnace reaches and holds the full 700 °C temperature to guarantee the total pyrolysis of CTAB and removal of any solvent residues.
By balancing thermal intensity with precise ramp control, you ensure the transition from a fragile gel to a robust, highly porous bioactive glass.
Summary Table:
| Process Phase | Primary Function | Key Parameter | Outcome |
|---|---|---|---|
| Template Removal | Pyrolysis of CTAB organic agents | High Temperature (700 °C) | Revealed mesoporous channels & high surface area |
| Phase Transition | Gel-to-glass silicate condensation | Precise Thermal Energy | Stable, amorphous bioactive glass structure |
| Heating Ramp | Controlled organic decomposition | Slow Rate (2 °C/min) | Prevention of structural collapse or agglomeration |
| Thermal Holding | Removal of residual toxins/solvents | Duration at Peak Temp | High purity material suitable for medical use |
Elevate Your Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing mesoporous bioactive glass. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to provide the exact ramp rates and temperature uniformity your research demands. Whether you need a standard lab furnace or a fully customizable high-temp solution, our equipment ensures structural integrity and purity for your most sensitive applications.
Ready to optimize your calcination process? Contact KINTEK today for a custom solution!
Visual Guide
References
- Usanee Pantulap, Aldo R. Boccaccini. Hydroxycarbonate apatite formation, cytotoxicity, and antibacterial properties of rubidium-doped mesoporous bioactive glass nanoparticles. DOI: 10.1007/s10934-023-01546-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of a high-temperature box-type resistance furnace in rGO synthesis? Optimize Your Carbonization
- What are the main applications of muffle furnaces? Unlock Clean, High-Temp Processing for Your Lab
- What are the core functions of a muffle furnace in the annealing process of SnO2 films? Optimize Your TCO Performance
- How are muffle furnaces utilized in dental labs? Essential for Precision Dental Restorations
- How does the use of gaskets or shims to adjust workpiece height affect the sintering process in a muffle furnace?
- How is a laboratory box resistance furnace utilized in the heat treatment and testing of high-speed steel samples?
- How do box resistance furnaces facilitate the optimization of mechanical properties in AlSi10Mg alloys? Expert Thermal Analysis
- What is a muffle furnace used for? Achieve Pure, High-Temperature Processing



















