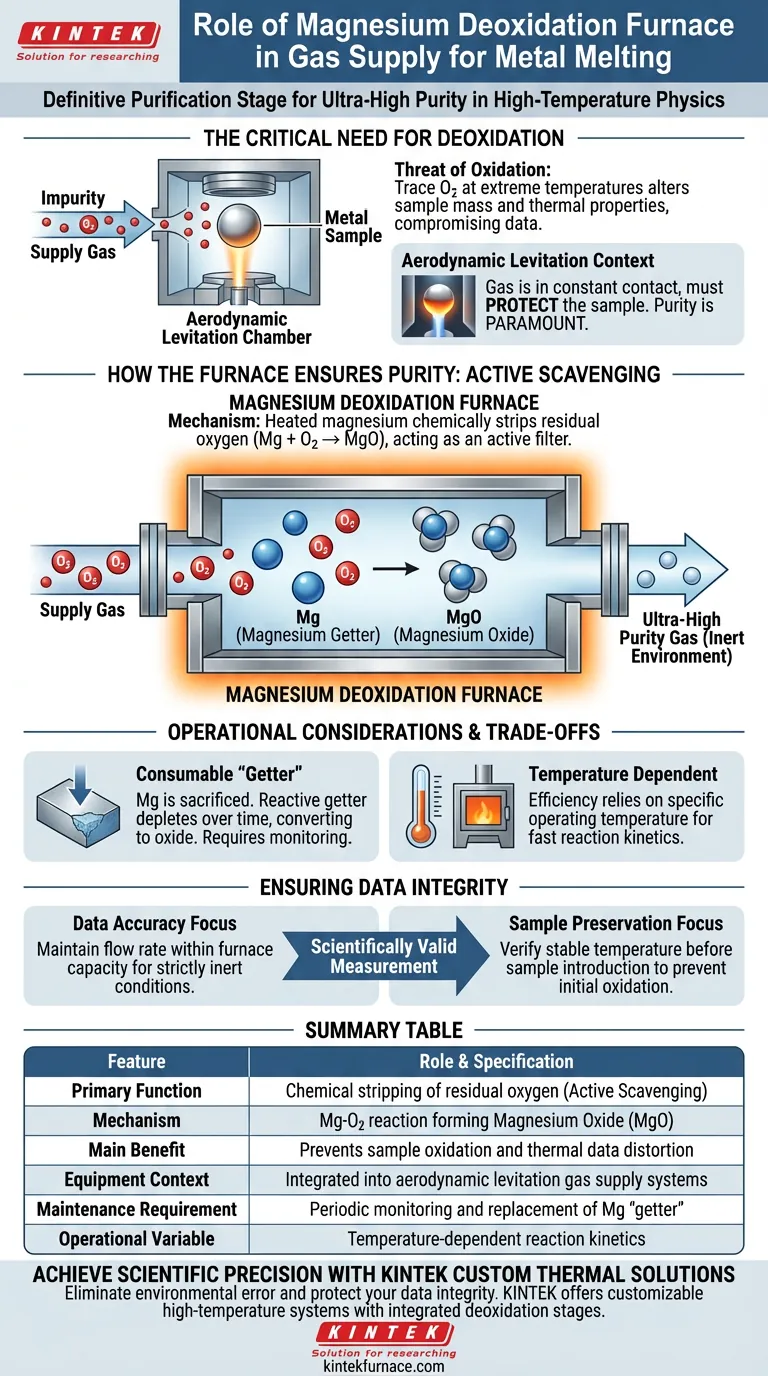
The magnesium deoxidation furnace serves as the definitive purification stage within a gas supply system. Its primary function is to chemically strip residual oxygen from the supply gas before it reaches the experiment. This ensures that the gas entering the aerodynamic levitation chamber achieves ultra-high purity, a non-negotiable requirement for precise metal melting heat measurements.
The Core Insight: In high-temperature metal physics, the gas supply is not just a tool for levitation; it is the chemical environment. The magnesium deoxidation furnace guarantees an ultra-low oxygen atmosphere, preventing the sample oxidation that would otherwise compromise thermal data and physical property measurements.

The Critical Need for Deoxidation
To understand the role of this furnace, you must first understand the vulnerability of the experiment.
The Threat of Oxidation
When measuring the melting heat of metals, the sample is heated to extreme temperatures. At these states, metals become highly reactive.
Even trace amounts of oxygen in the gas supply can lead to immediate surface oxidation. This chemical reaction alters the sample's mass and thermal properties, rendering the measurement data inaccurate.
The Role of Aerodynamic Levitation
These systems typically utilize an aerodynamic levitation chamber. In this setup, the metal sample is floated on a jet of gas to avoid contact with a container.
Because the gas is in constant, direct contact with the molten metal, its purity is paramount. The gas must do more than lift the sample; it must protect it.
How the Furnace Ensures Purity
The magnesium deoxidation furnace is an active filtration component, not a passive filter.
Active Chemical Scavenging
The furnace utilizes magnesium's high chemical affinity for oxygen. As the supply gas passes through the heated furnace, the magnesium reacts with any oxygen present.
This process effectively "traps" the oxygen by converting it into magnesium oxide.
Achieving Ultra-High Purity
Standard industrial gases often contain impurities that are acceptable for manufacturing but disastrous for scientific measurement.
The furnace polishes the gas to an ultra-high purity level. This creates the specific inert environment required to keep the molten metal sample pristine during the experiment.
Operational Considerations and Trade-offs
While essential for accuracy, the inclusion of a magnesium deoxidation furnace introduces specific maintenance requirements.
The Consumable Nature of the "Getter"
The magnesium within the furnace acts as a "getter"—it sacrifices itself to capture the oxygen.
Over time, the reactive magnesium is depleted as it converts to oxide. It is not an infinite resource and requires monitoring to ensure the deoxidation capability remains active.
Temperature Dependencies
The efficiency of the deoxidation process is temperature-dependent.
The furnace must be maintained at a specific operating temperature to ensure the reaction kinetics are fast enough to strip the oxygen from the flowing gas stream.
Ensuring Data Integrity in Your Experiments
The deoxidation furnace is the difference between a rough estimate and a scientifically valid measurement.
- If your primary focus is Data Accuracy: Ensure the gas flow rate does not exceed the furnace's capacity to react with impurities, maintaining strictly inert conditions.
- If your primary focus is Sample Preservation: verify that the furnace operating temperature is stable before introducing the sample to prevent initial surface oxidation.
By treating the gas supply as a critical variable and purifying it actively, you eliminate environmental contamination as a source of error in your thermal analysis.
Summary Table:
| Feature | Role & Specification |
|---|---|
| Primary Function | Chemical stripping of residual oxygen (Active Scavenging) |
| Mechanism | Magnesium-oxygen reaction forming Magnesium Oxide (MgO) |
| Main Benefit | Prevents sample oxidation and thermal data distortion |
| Equipment Context | Integrated into aerodynamic levitation gas supply systems |
| Maintenance Requirement | Periodic monitoring and replacement of magnesium "getter" |
| Operational Variable | Temperature-dependent reaction kinetics |
Achieve Scientific Precision with KINTEK Custom Thermal Solutions
In high-temperature metal physics, your gas environment is just as critical as your furnace. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the most demanding research applications. Our high-temperature furnaces are fully customizable to integrate advanced deoxidation and purification stages, ensuring the ultra-low oxygen atmospheres necessary for your aerodynamic levitation and melting experiments.
Eliminate environmental error and protect your data integrity today.
Contact KINTEK for a Custom Solution
Visual Guide
References
- Kanta Kawamoto, Hidekazu Kobatake. Development of Heat-of-fusion Measurement for Metals Using a Closed-type Aerodynamic Levitator. DOI: 10.2355/isijinternational.isijint-2024-053
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the automotive industry applications of furnace brazing? Enhance Vehicle Performance with Strong, Leak-Proof Joints
- What are the environmental benefits of vacuum furnaces? Achieve Zero Emissions & High Efficiency
- Why is graphite the material of choice for vacuum furnaces? Unmatched Performance in Extreme Heat
- What is vacuum brazing and how does it work? Achieve High-Strength, Clean Joints for Complex Assemblies
- Why is vacuum sublimation preferred over recrystallization for MTO? Achieve High-Purity Methyltrioxorhenium
- Why Multiple Vacuum & Argon Cycles for High-Entropy Alloys? Achieve Peak Purity & Chemical Precision!
- Why is an industrial vacuum drying oven essential for ceramic precursors? Preserve Material Purity and Stoichiometry
- Why is removing oxygen important in a vacuum furnace? Prevent Oxidation for Superior Metal Quality



















