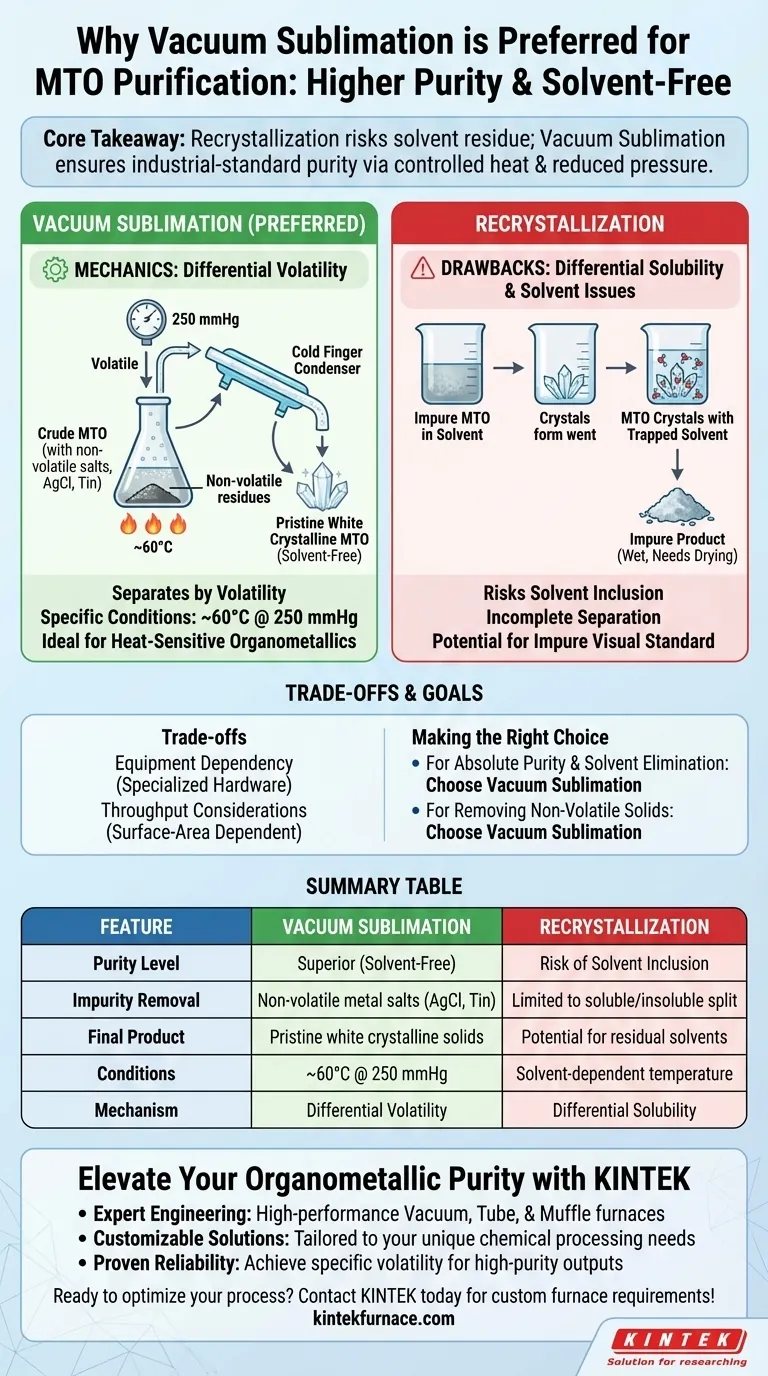
Vacuum sublimation is the preferred method for purifying Methyltrioxorhenium (MTO) because it yields a higher purity product free from solvent contamination. By exploiting the specific volatility of MTO, this process effectively separates the compound from non-volatile metal salts and solid residues. Unlike recrystallization, which often traps solvent molecules within the crystal lattice, vacuum sublimation produces pristine, white crystalline solids directly.
Core Takeaway: Recrystallization introduces the risk of solvent residue and incomplete separation. Vacuum sublimation circumvents this by utilizing controlled heat and reduced pressure to isolate MTO from non-volatile by-products, ensuring an industrial standard of purity.

The Mechanics of Purification
Exploiting Differential Volatility
The primary advantage of vacuum sublimation is its ability to separate compounds based on their tendency to vaporize. MTO is volatile, meaning it can transition directly from a solid to a gas under the right conditions.
In contrast, the impurities typically found in crude MTO, such as silver chloride or tin by-products, are non-volatile solid residues. When the mixture is heated, the MTO vaporizes and leaves these heavy metal salts behind.
Precise Operational Conditions
This process relies on specific environmental controls to be effective. The standard protocol involves heating the material to approximately 60°C under a reduced pressure of 250 mmHg.
These conditions are mild enough to protect the integrity of the compound while ensuring efficient sublimation. This makes it the standard industrial method for refining heat-sensitive organometallic compounds.
The Drawbacks of Recrystallization
The Issue of Solvent Residue
Recrystallization relies on dissolving a solid in a solvent and then precipitating it back out. A major downside of this method for MTO is the potential for solvent inclusion.
During the formation of the crystal lattice, solvent molecules can become trapped inside. This results in an impure product that requires further processing to dry completely.
Visual Indicators of Purity
The goal of MTO purification is to obtain a specific visual standard: a white crystalline product.
Recrystallization often fails to achieve this level of clarity due to the aforementioned trapped solvents or remaining dissolved impurities. Vacuum sublimation produces this high-purity white solid in a single step.
Understanding the Trade-offs
Equipment Dependency
While sublimation offers superior purity, it requires more specialized hardware than recrystallization. You must have access to a reliable vacuum system capable of maintaining 250 mmHg and precise temperature control.
Throughput Considerations
Sublimation is a surface-area-dependent process. Unlike recrystallization, which occurs in the bulk volume of a solution, sublimation rates are limited by the surface area of the crude material and the condensing surface.
Making the Right Choice for Your Goal
When deciding on a purification strategy for MTO or similar organometallics, consider your specific purity requirements.
- If your primary focus is absolute purity: Choose vacuum sublimation to eliminate the risk of solvent entrapment and ensure the removal of metal salts.
- If your primary focus is removing non-volatile solids: Choose vacuum sublimation, as residues like silver chloride will not transfer to the final product.
By controlling pressure and temperature, you transform a complex separation challenge into a straightforward phase change, securing the highest quality product available.
Summary Table:
| Feature | Vacuum Sublimation | Recrystallization |
|---|---|---|
| Purity Level | Superior (Solvent-Free) | Risk of Solvent Inclusion |
| Impurity Removal | Non-volatile metal salts (AgCl, Tin) | Limited to soluble/insoluble split |
| Final Product | Pristine white crystalline solids | Potential for residual solvents |
| Conditions | ~60°C @ 250 mmHg | Solvent-dependent temperature |
| Mechanism | Differential Volatility | Differential Solubility |
Elevate Your Organometallic Purity with KINTEK
Precise control over temperature and pressure is the cornerstone of successful MTO purification. At KINTEK, we provide the advanced hardware necessary for industrial-grade sublimation, ensuring your sensitive materials remain pristine and free from contamination.
Our Value to You:
- Expert Engineering: Backed by specialist R&D and manufacturing, we deliver high-performance Vacuum, Tube, and Muffle furnace systems.
- Customizable Solutions: All our lab high-temp furnaces are tailored to meet your unique chemical processing needs.
- Proven Reliability: Achieve the specific volatility required for high-purity crystalline outputs every time.
Ready to optimize your purification process? Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Joanna Malarz, Katarzyna Leszczyńska-Sejda. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. DOI: 10.3390/cryst15080717
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- How does a vacuum system facilitate antimony-tellurium alloy distillation? Maximize Purity with Low-Temp Separation
- Why is a programmable vacuum sintering furnace required? Ensure Superior Feldspathic to Zirconia Bonding
- Which performance characteristics of ultra-high temperature graphite furnaces are essential for Boron Carbide sintering?
- What are the primary functions of a laboratory drying oven in banana peel activated carbon? Optimize Every Process Step
- What is the objective of vacuum drying in NaF–Na3AlF6 electrolytes? Ensure High-Purity Molten Salt Performance
- Why is a two-step cooling system necessary for titanium hydrogenation? Protect Your Alloy’s Integrity
- What is the primary function of a vacuum drying oven in the synthesis of H2bdt organic ligands? Protect Your Purity.
- What are the technical advantages of using a vacuum environment for drying g-C3N4/Bi2WO6 catalysts?



















