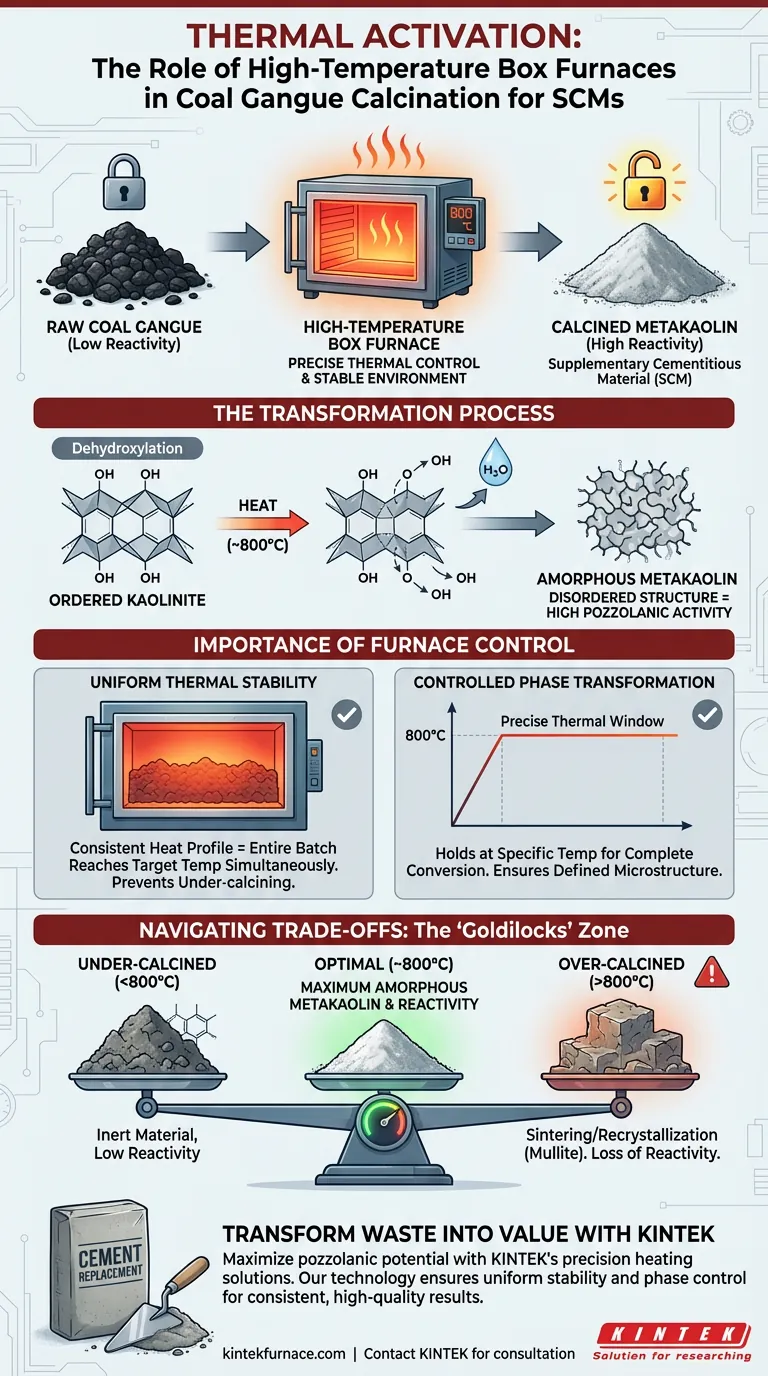
The primary role of a high-temperature box furnace is to create a stable thermal environment, specifically around 800 °C, that chemically activates coal gangue. This heat treatment facilitates the dehydroxylation of kaolinite, transforming the inert mineral structure into amorphous metakaolin, which is essential for its use as a partial cement replacement.
Raw coal gangue possesses low chemical reactivity, making it unsuitable for construction use in its natural state. The box furnace serves as a critical activation tool, utilizing precise thermal control to alter the material’s phase structure and unlock the high pozzolanic activity required for supplementary cementitious materials.
The Mechanism of Thermal Activation
The utility of a box furnace lies in its ability to drive specific chemical changes that do not occur at ambient temperatures.
Dehydroxylation of Kaolinite
The core reaction driven by the furnace is dehydroxylation. When the furnace maintains a temperature of approximately 800 °C, it forces the removal of hydroxyl groups (-OH) from the kaolinite minerals present within the coal gangue.
Creation of Amorphous Metakaolin
As the hydroxyl groups are removed, the crystal structure of the material changes. The ordered kaolinite transforms into amorphous metakaolin. This disordered, amorphous structure is highly energetic and reactive, which is the defining characteristic of a good pozzolan.
Enabling Cement Replacement
Once activated, the material can react with calcium hydroxide (a byproduct of cement hydration). This reaction creates additional binding compounds, allowing the treated coal gangue to partially replace Portland cement in concrete and mortar.
The Importance of Environment Control
While the temperature is the catalyst, the stability provided by the box furnace is the control mechanism that ensures quality.
Uniform Thermal Stability
A high-temperature box furnace is designed to provide a consistent heat profile. This ensures that the entire batch of coal gangue reaches the target temperature simultaneously. Uniform heating prevents parts of the material from remaining under-calcined (inert) while others become over-calcined.
Controlled Phase Transformation
The transition to metakaolin requires a specific thermal window. The furnace allows operators to hold the material at the precise temperature required for the phase transformation to complete, ensuring the final product possesses a consistent, well-defined microstructure.
Understanding the Trade-offs
Achieving high pozzolanic activity requires navigating specific thermal risks.
The Risk of Overheating
While heating is essential, excessive heat is detrimental. If the furnace temperature significantly exceeds the 800 °C range, the material may begin to sinter or recrystallize into stable, non-reactive phases (such as mullite). This destroys the amorphous structure and drastically reduces the material's reactivity.
Energy vs. Activation Balance
The box furnace process is energy-intensive. The goal is to input just enough energy to achieve full dehydroxylation without wasting energy on higher temperatures that offer no additional benefit or potentially degrade the material.
Making the Right Choice for Your Goal
When utilizing a high-temperature box furnace for coal gangue calcination, your operational parameters should align with your specific material requirements.
- If your primary focus is maximum reactivity: strictly maintain the temperature around 800 °C to maximize the yield of amorphous metakaolin and avoid recrystallization.
- If your primary focus is process consistency: prioritize a furnace with superior insulation and temperature uniformity to ensure the entire batch undergoes identical dehydroxylation.
The box furnace is not merely a heater; it is a precision instrument that turns industrial waste into a valuable construction resource through controlled phase transformation.
Summary Table:
| Feature | Impact on Coal Gangue Calcination |
|---|---|
| Optimal Temperature | ~800°C for maximum dehydroxylation |
| Mineral Phase Change | Converts inert kaolinite to amorphous metakaolin |
| Thermal Stability | Ensures uniform activation and prevents under-calcining |
| Risk Mitigation | Precise control avoids overheating and recrystallization |
| End Product | High-reactivity pozzolan for cement replacement |
Transform Industrial Waste into Value with KINTEK
Maximize the pozzolanic potential of your materials with precision heating solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temperature furnaces—all fully customizable to meet your unique calcination requirements.
Whether you are optimizing coal gangue activation or developing advanced cementitious materials, our technology ensures the uniform thermal stability and phase control you need for consistent, high-quality results.
Ready to elevate your material processing? Contact KINTEK today to consult with our specialists!
Visual Guide
References
- Wenguang Zhang, Sun Jin-Feng. Influence of Curing Temperature on the Performance of Calcined Coal Gangue–Limestone Blended Cements. DOI: 10.3390/ma17081721
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What benefits do box furnaces offer in terms of material properties? Achieve Superior Material Control and Performance
- Why is a laboratory high-temperature oven necessary for heterogeneous decatungstate catalysts? Ensure Structural Fixation
- Why is a high-precision high-temperature furnace required for chemical activation? Ensure Superior Carbon Quality
- Why is a box furnace required for the calcination of hydroxide precursors? Master P2-Type Oxide Synthesis
- How does secondary calcination in a high-temperature furnace regenerate CLDH clay? Unlock the Memory Effect
- How does the temperature control system work in a muffle furnace? Ensure Precise Heating for Your Lab
- Why is working temperature the most crucial factor when choosing a muffle furnace? Ensure Your Process Success with the Right Heat
- How does the muffle design benefit sample processing? Ensure Purity and Precision in Your Lab



















