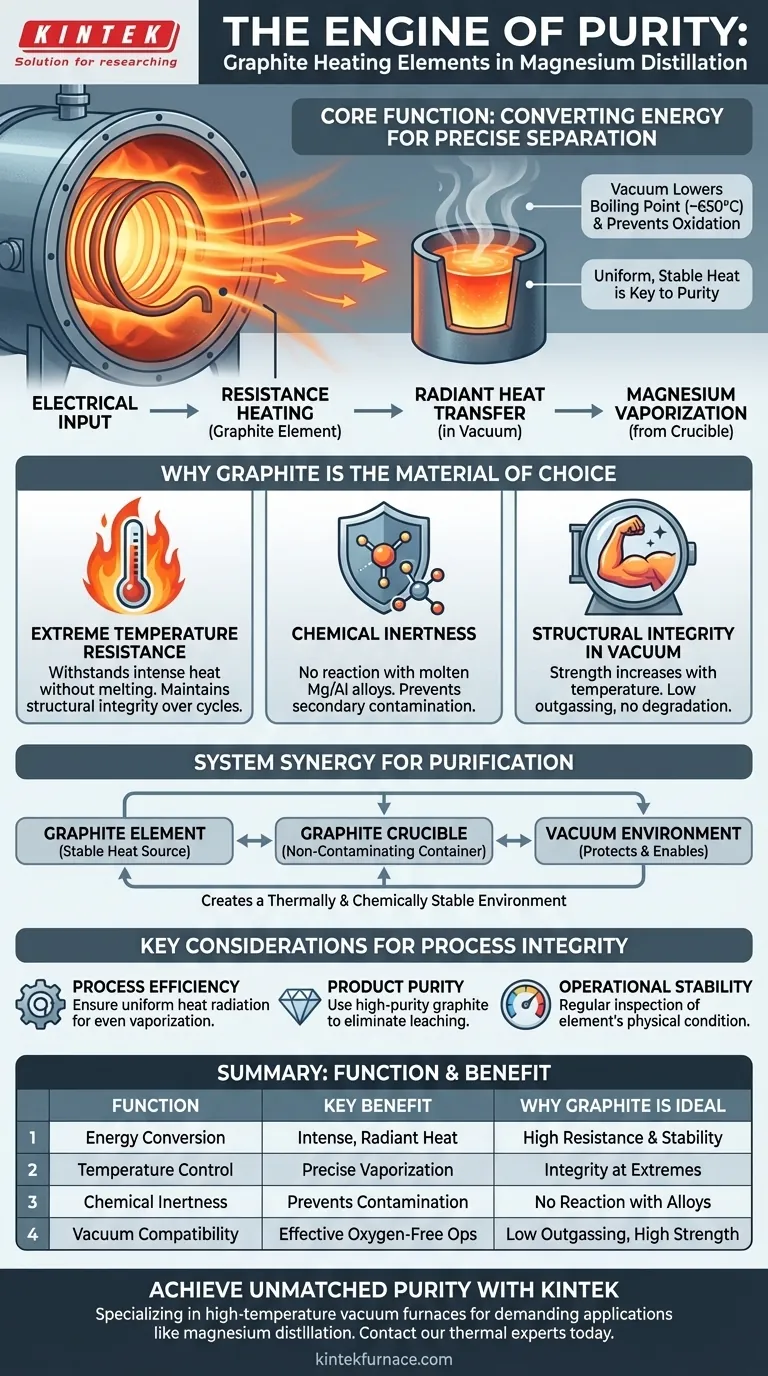
In a vacuum furnace for magnesium distillation, the graphite heating element is the engine of the purification process. Its fundamental role is to convert electrical energy into stable, high-temperature thermal energy. This radiant heat is directed at a graphite crucible, causing the crude magnesium inside to vaporize so it can be separated from less volatile impurities, a critical step in achieving high purity.
The use of a graphite heating element is not merely a choice of material, but a strategic decision. Its unique ability to withstand extreme temperatures, radiate heat uniformly, and remain chemically inert is the key to unlocking the precise and stable thermal control required for effective magnesium distillation.

The Core Function: From Electricity to Purified Metal
Understanding how the heating element operates within the system reveals why it is so critical. The process is a careful orchestration of physics and material science, all taking place within a controlled vacuum.
Energy Conversion and Heat Transfer
The graphite element works on the principle of resistance heating. When a high electrical current passes through it, the graphite's natural resistance converts this electrical energy into intense heat.
Because this occurs in a vacuum, heat is transferred primarily through thermal radiation, not convection. The element glows, radiating energy directly to the graphite crucible containing the magnesium alloy.
Enabling Precise Temperature Control
The success of distillation hinges on maintaining a specific temperature. The magnesium needs to vaporize, but the impurities must remain behind.
Graphite's excellent structural stability at high temperatures allows the system to hold a precise and unwavering temperature, ensuring a clean and selective separation of the target metal.
The Importance of a Vacuum Environment
The vacuum is essential for two reasons. First, it lowers the boiling point of magnesium, allowing distillation to occur at a more manageable temperature (around 650°C). Second, it removes oxygen, preventing oxidation of both the molten metal and the heating element itself.
Why Graphite is the Material of Choice
Other materials could generate heat, but graphite possesses a unique combination of properties that make it perfectly suited for this demanding application. Its selection is central to the process's efficiency and the final product's purity.
Extreme Temperature Resistance
Unlike most metals which melt, graphite can withstand extremely high temperatures without losing its structural integrity. This ensures the heating element remains stable and reliable throughout many operational cycles.
Chemical Inertness
High-purity graphite is chemically stable and does not react with the molten magnesium-aluminum alloy. This inertness is crucial, as it prevents the heating element—or the crucible—from becoming a source of secondary contamination.
Structural Integrity in a Vacuum
Graphite's physical strength actually increases with temperature up to a certain point. This makes it exceptionally well-suited for the harsh, oxygen-free environment of a vacuum furnace, where other materials might degrade or release unwanted gases (outgassing).
Understanding the System's Interplay
The heating element does not work in isolation. Its effectiveness is tied directly to the other components of the furnace, creating a synergistic system designed for one purpose: purification.
The Element and the Crucible
The system uses both a graphite heating element and a graphite crucible. This shared material choice is intentional. The element provides the stable, non-contaminating heat source, while the crucible provides a stable, non-contaminating container. Together, they create a thermally and chemically stable environment.
The Element and the Vacuum
The vacuum protects the graphite element from oxidation, allowing it to function at high temperatures for extended periods. In turn, the element's ability to operate cleanly without outgassing helps maintain the integrity of the vacuum, which is essential for the distillation process itself.
Key Considerations for Process Integrity
To leverage this technology effectively, one must consider how the heating element impacts core operational goals.
- If your primary focus is process efficiency: Ensure the heating element is designed for uniform heat radiation to minimize energy waste and guarantee even vaporization from the crucible.
- If your primary focus is product purity: Verify that both the heating element and the crucible are made from high-purity graphite to eliminate any risk of chemical leaching or contamination.
- If your primary focus is operational stability: Implement regular inspections of the heating element's physical condition, as its structural integrity is the foundation of the entire thermal control system.
Ultimately, the graphite heating element is the enabling technology that transforms a furnace into a precision instrument for metal purification.
Summary Table:
| Function | Key Benefit | Why Graphite is Ideal |
|---|---|---|
| Energy Conversion | Converts electricity to intense, radiant heat | High resistance and temperature stability |
| Temperature Control | Enables precise vaporization of magnesium | Maintains structural integrity at extreme temperatures |
| Chemical Inertness | Prevents contamination of the final product | Does not react with molten magnesium alloys |
| Vacuum Compatibility | Operates effectively in an oxygen-free environment | Low outgassing and increased strength in a vacuum |
Achieve Unmatched Purity in Your Metal Distillation Processes
Precise thermal control is the foundation of effective magnesium purification. The right furnace system, with a high-performance graphite heating element at its core, is critical for maximizing yield, ensuring product purity, and maintaining operational stability.
At KINTEK, we specialize in designing and manufacturing high-temperature vacuum furnaces tailored for demanding applications like magnesium distillation. Our expertise in material science and thermal engineering ensures your furnace delivers:
- Superior Temperature Uniformity: For consistent and efficient vaporization.
- Guaranteed Chemical Inertness: Using high-purity components to protect your product.
- Robust and Reliable Operation: Engineered for long-term stability in harsh environments.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique process needs.
Ready to optimize your distillation process? Contact our thermal experts today to discuss how a KINTEK vacuum furnace can be the engine of your success.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
People Also Ask
- Why are graphite fixtures and holders important in vacuum furnaces? Unlock Precision & Durability
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- What is the mechanism and effect of post-annealing NiTi thin films in a vacuum furnace? Unlock Superelasticity
- Why is graphite cost-effective for vacuum furnaces? Maximize Long-Term ROI & Efficiency
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing



















