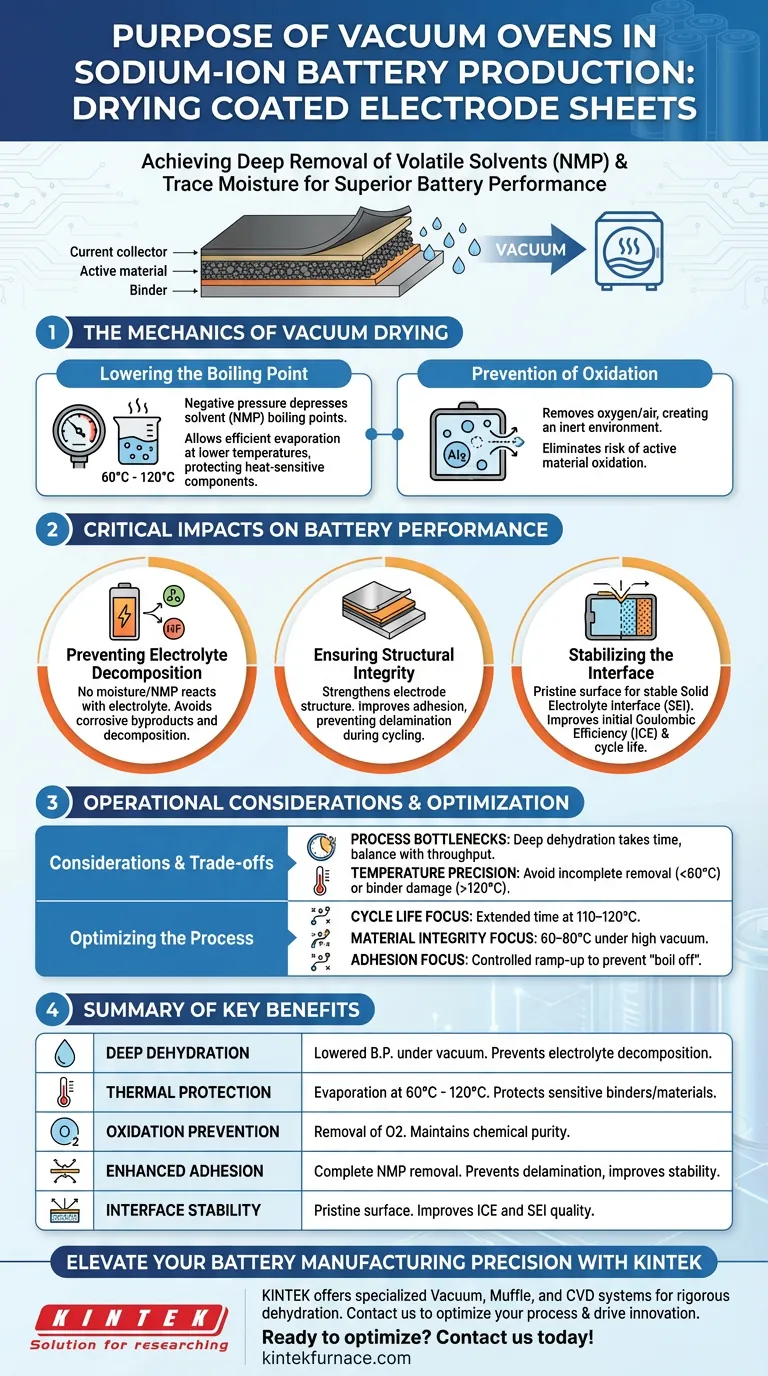
The primary purpose of using a vacuum oven in sodium-ion battery production is to achieve the deep removal of volatile solvents, specifically N-Methyl-2-pyrrolidone (NMP), and trace moisture from coated electrode sheets.
By operating under negative pressure, the oven significantly lowers the boiling point of these liquids. This allows for complete evaporation at relatively low temperatures—typically between 60 °C and 120 °C—ensuring the electrode is thoroughly dried without subjecting the active materials to thermal degradation.
Core Insight: While standard heating removes surface liquids, vacuum drying is the only reliable method to extract deep-seated residual solvents and adsorbed water. This step is non-negotiable for sodium-ion batteries, as even microscopic traces of moisture can trigger electrolyte decomposition, drastically reducing the battery's safety and cycle life.
The Mechanics of Vacuum Drying
Lowering the Boiling Point
The central advantage of a vacuum oven is the manipulation of pressure. By creating a vacuum environment, the system depresses the boiling point of solvents like NMP.
This allows manufacturers to drive off solvents efficiently at lower thermal thresholds (often 110 °C or lower).
This protects temperature-sensitive components in the electrode slurry from heat damage while ensuring rapid evaporation.
Prevention of Oxidation
Drying at high temperatures in standard air can lead to the oxidation of active materials.
Vacuum ovens eliminate this risk by removing the air (and oxygen) from the chamber.
This creates an inert environment where the electrode sheets can be dehydrated without chemically reacting with the atmosphere.
Critical Impacts on Battery Performance
Preventing Electrolyte Decomposition
Sodium-ion battery materials are highly sensitive to moisture and chemical impurities.
If residual NMP or water remains in the electrode, it reacts with the electrolyte once the battery is assembled.
This reaction often produces corrosive byproducts (similar to HF generation in lithium batteries) and leads to the decomposition of the electrolyte, compromising the cell's internal chemistry.
Ensuring Structural Integrity
Thorough drying strengthens the physical structure of the electrode.
Removing residues improves the adhesion between the active material layer and the current collector (aluminum foil).
Stronger adhesion prevents delamination during the battery's expansion and contraction cycles, directly contributing to long-term stability.
Stabilizing the Interface
A contaminant-free electrode is required to form a stable Solid Electrolyte Interface (SEI) film.
The vacuum process ensures that the surface chemistry is pristine, which improves the Initial Coulombic Efficiency (ICE).
Without this deep drying, side reactions would destabilize the SEI, leading to rapid capacity fade.
Operational Considerations and Trade-offs
Process Bottlenecks
Vacuum drying is rarely an instantaneous process; it is often the bottleneck in electrode fabrication.
Achieving "deep dehydration" frequently requires extended processing times, sometimes lasting overnight.
Manufacturers must balance the need for absolute dryness against production throughput speed.
Temperature Precision
While vacuum lowers the required temperature, the thermal setting must still be precise.
If the temperature is too low (e.g., significantly below 60 °C), NMP removal may be incomplete despite the vacuum.
Conversely, excessively high temperatures (above 120 °C) risk damaging the polymer binders that hold the electrode together.
Optimizing the Drying Process
To ensure the highest quality sodium-ion electrodes, align your drying parameters with your specific performance goals.
- If your primary focus is Cycle Life: Prioritize extended drying times at moderate temperatures (110–120 °C) to eliminate every trace of moisture that could degrade the electrolyte.
- If your primary focus is Material Integrity: Utilize lower-temperature settings (60–80 °C) under high vacuum to prevent oxidation of sensitive active materials.
- If your primary focus is Adhesion: Ensure the drying ramp-up is controlled to prevent solvent "boil off" that can disrupt the binder distribution and weaken the coating.
Ultimately, the vacuum oven is not just a drying tool; it is a critical instrument for ensuring the electrochemical purity required for a safe, long-lasting battery.
Summary Table:
| Key Benefit | Mechanism | Impact on Battery |
|---|---|---|
| Deep Dehydration | Lowered boiling point under vacuum | Prevents electrolyte decomposition and HF formation |
| Thermal Protection | Evaporation at 60°C - 120°C | Protects temperature-sensitive binders and materials |
| Oxidation Prevention | Removal of oxygen/air from chamber | Maintains chemical purity of the active materials |
| Enhanced Adhesion | Complete removal of residual NMP | Prevents delamination and improves cycle stability |
| Interface Stability | Pristine surface chemistry | Improves Initial Coulombic Efficiency (ICE) and SEI film quality |
Elevate Your Battery Manufacturing Precision with KINTEK
Don't let residual moisture compromise your sodium-ion battery performance. Backed by expert R&D and manufacturing, KINTEK offers specialized Vacuum, Muffle, and CVD systems designed to meet the rigorous dehydration needs of electrode production. Our lab high-temp furnaces are fully customizable to ensure your materials achieve peak structural integrity and electrochemical purity.
Ready to optimize your drying process? Contact us today to discuss your unique needs and see how our expert-engineered solutions can drive your battery innovation forward.
Visual Guide
References
- Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism. DOI: 10.3390/nano15120893
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- How does a Grain Boundary Diffusion (GBD) heat treatment furnace improve the performance of high-grade magnets?
- What components make up the vacuum system in a vacuum furnace? Explore the Key Parts for Optimal Performance
- What are the advantages of graphite furnace? Achieve Unmatched High-Temperature Performance
- Where are vacuum furnaces used? Critical Applications in Aerospace, Medical, and Electronics
- What processes can vacuum carburizing furnaces perform? Unlock Versatile Heat Treatment Solutions
- What environmental benefits do continuous vacuum furnaces provide? Achieve Zero Emissions and High Efficiency
- What are the general operational features of a vacuum furnace? Achieve Superior Material Purity & Precision
- What role does a vacuum chamber play in the Flash Joule Heating (FJH) process for LIG? Master Graphene Synthesis



















