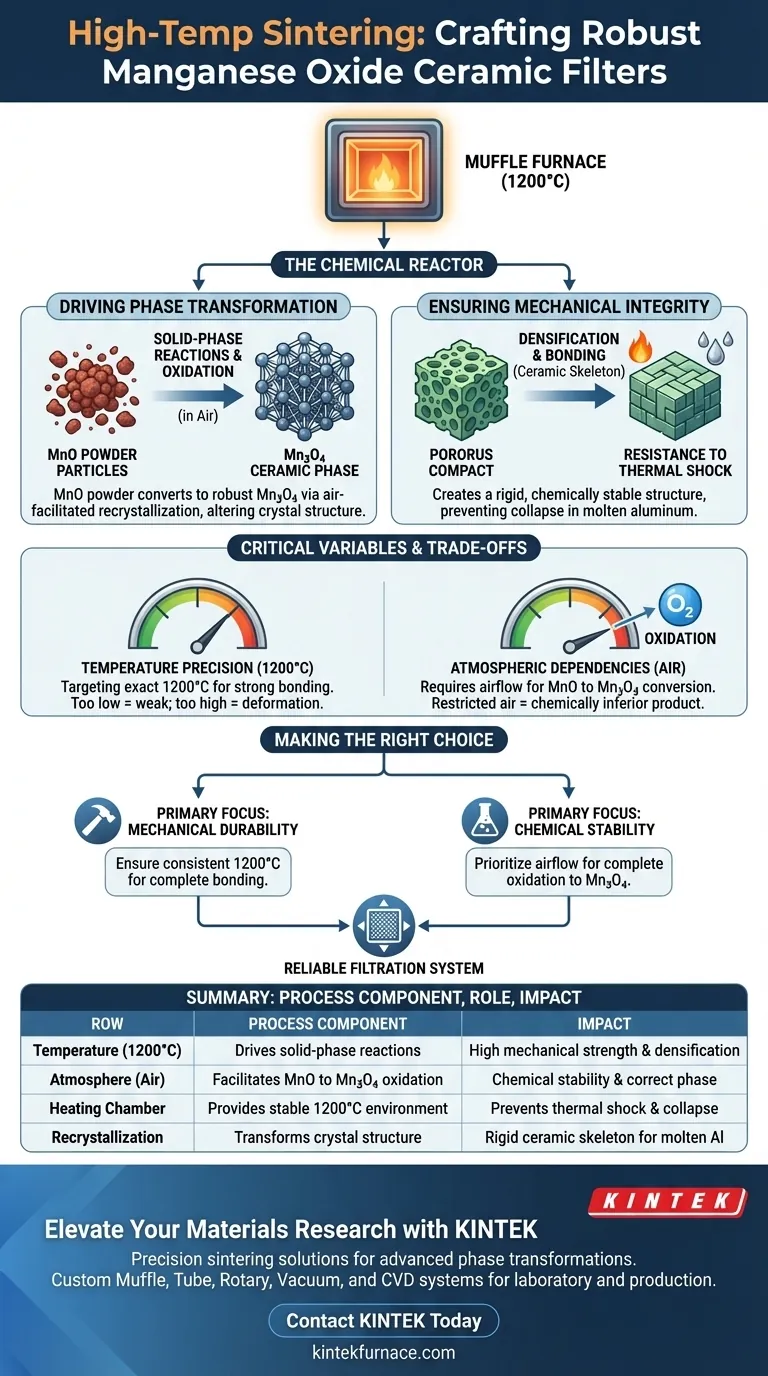
The high-temperature muffle furnace acts as a critical chemical reactor, not just a heat source. Its primary purpose in this specific application is to provide a stable 1200°C environment that drives the oxidation and recrystallization of manganese oxide (MnO) compacts. This process transforms the raw material into a robust ceramic phase dominated by Mn3O4, ensuring the final substrate has the necessary mechanical strength to filter molten aluminum without collapsing.
The core function of the sintering process is to convert a fragile powder compact into a rigid, chemically stable structure. Without the specific phase transformation induced by the furnace, the substrate would fail catastrophically under industrial filtration conditions.

Driving Phase Transformation
The furnace does not simply dry or harden the material; it fundamentally alters its chemical composition and crystal structure.
Solid-Phase Reactions
At 1200°C, the furnace facilitates solid-phase reactions.
This allows the material components to react chemically without fully melting. This creates strong bonds between particles that were previously only loosely compacted.
Oxidation and Recrystallization
The process relies on the presence of air within the furnace chamber.
During sintering, the manganese oxide (MnO) undergoes oxidation. This triggers recrystallization, shifting the material's composition to a ceramic phase dominated by Mn3O4. This specific phase is essential for the material's final properties.
Ensuring Mechanical Integrity
The ultimate goal of the heating process is to create a filter that can survive an aggressive industrial environment.
Densification and Bonding
The heat drives the transition from a "green" (unfired) compact to a sintered ceramic.
This involves physicochemical bonding that acts as a ceramic skeleton. It locks the structure in place, significantly increasing the mechanical strength of the substrate.
Resistance to Thermal Shock
The most critical performance metric is stability during use.
The sintered Mn3O4 structure is designed to withstand contact with high-temperature aluminum melts. If the sintering is incomplete, the substrate would lack the structural stability required and would likely collapse during the filtration process.
Critical Process Variables and Trade-offs
While the furnace enables high performance, the process requires strict control to avoid defects.
Temperature Precision
The specific target of 1200°C is not arbitrary.
Deviating significantly from this temperature can result in incomplete solid-phase reactions. Too low, and the bond is weak; too high, and you risk unwanted deformation or melting.
Atmospheric Dependencies
Because the process involves oxidation (converting MnO to Mn3O4), the atmosphere inside the furnace is a critical variable.
Unlike sintering processes that require inert gases, this process demands air. Restricting airflow in the muffle furnace could inhibit the necessary oxidation, leading to a chemically inferior product.
Making the Right Choice for Your Goal
Optimizing the sintering profile depends on which failure mode you are trying to prevent in your final product.
- If your primary focus is mechanical durability: Ensure the furnace maintains a consistent 1200°C to guarantee complete particle bonding and structural density.
- If your primary focus is chemical stability: Prioritize airflow and atmospheric control to ensure the complete oxidation and recrystallization into the Mn3O4 phase.
The muffle furnace is the bridge between a raw chemical compound and a functional industrial tool, defining the ultimate reliability of the filtration system.
Summary Table:
| Process Component | Role in Sintering | Impact on Final Product |
|---|---|---|
| Temperature (1200°C) | Drives solid-phase reactions | Ensures high mechanical strength and densification |
| Atmosphere (Air) | Facilitates MnO to Mn3O4 oxidation | Guarantees chemical stability and correct phase formation |
| Heating Chamber | Provides stable 1200°C environment | Prevents thermal shock and structural collapse during filtration |
| Recrystallization | Transforms crystal structure | Creates a rigid ceramic skeleton for molten aluminum contact |
Elevate Your Materials Research with KINTEK
Precision sintering is the difference between a fragile compact and a high-performance industrial ceramic. KINTEK provides the industry-leading thermal solutions required for advanced phase transformations.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique laboratory or production needs. Whether you are optimizing manganese oxide oxidation or developing new ceramic substrates, our furnaces deliver the temperature precision and atmospheric control critical to your success.
Ready to optimize your high-temperature processes? Contact KINTEK today to discuss your custom furnace requirements with our technical team!
Visual Guide
References
- Hanka Becker, Andreas Leineweber. Reactive Interaction and Wetting of Fe‐ and Mn‐Containing, Secondary AlSi Alloys with Manganese Oxide Ceramic Filter Material for Fe Removal. DOI: 10.1002/adem.202500636
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How is a laboratory muffle furnace utilized during the debinding stage of HAp green bodies? Precision Thermal Control
- What materials are prohibited from being placed in a high-temperature muffle furnace? Ensure Safety and Protect Your Equipment
- How is a muffle furnace utilized in the annealing of bismuth-lead-borosilicate glass? Mastering Stress Relief
- How does a high-precision furnace enhance EIS testing for niobium-doped titanium dioxide? Achieve Accurate Material Data
- What is the role of a laboratory drying oven or hot plate in slurry processing? Optimize Composite Material Quality
- What is the core role of a muffle furnace in the synthesis of calcium oxide from eggshells? Achieve High-Purity CaO
- What is the role of a laboratory high-temperature muffle furnace in the carbonization of sunflower seed husks?
- What role does a muffle furnace play in the curing process of GaN and TiO2? Optimize Your Photoanode Sintering



















