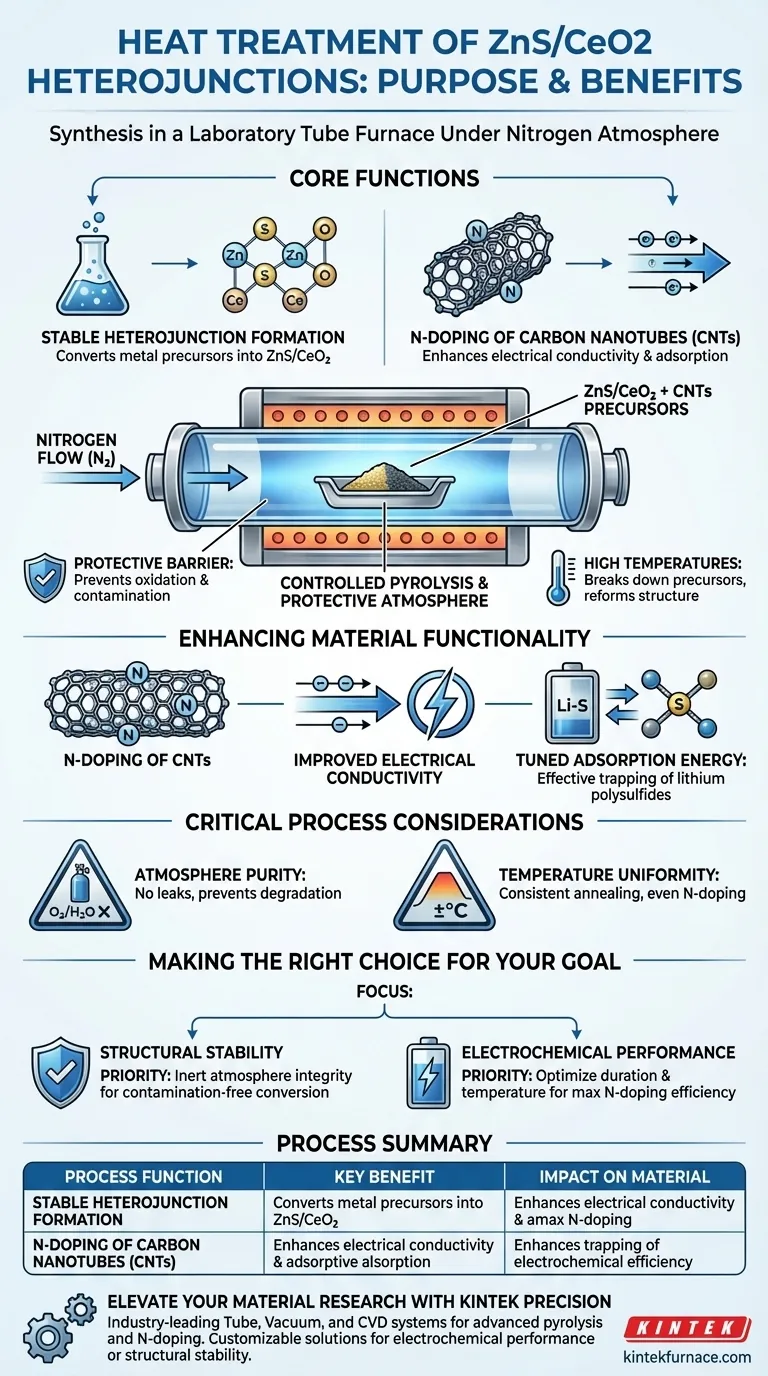
The heat treatment in a nitrogen atmosphere serves two critical functions: converting metal precursors into stable ZnS/CeO2 heterojunctions and simultaneously enabling the nitrogen-doping of Carbon Nanotubes (CNTs). The laboratory tube furnace provides a controlled pyrolysis environment, protecting the material from unwanted reactions during high-temperature annealing.
The nitrogen atmosphere acts as both a protective shield for structural formation and an active agent for chemical enhancement, directly improving electrical conductivity and lithium polysulfide adsorption.

Establishing the Synthesis Environment
Controlled Pyrolysis
The primary mechanical function of the tube furnace in this context is to facilitate controlled pyrolysis.
High temperatures are required to break down metal precursors and reform them into the desired ZnS/CeO2 heterojunction structure.
Protective Atmosphere
Using a nitrogen atmosphere creates a protective barrier around the sample.
This prevents uncontrolled oxidation or contamination from ambient air, ensuring the precursors convert strictly into the intended stable heterojunctions.
Enhancing Material Functionality
Nitrogen-Doping of CNTs
Beyond stabilizing the structure, the nitrogen atmosphere plays an active role in modifying the carbon support matrix.
The process enables the nitrogen-doping of Carbon Nanotubes (CNTs) present in the composite.
Improving Conductivity
This doping process significantly enhances the electrical conductivity of the material.
By introducing nitrogen atoms into the carbon lattice, the electronic properties of the CNTs are optimized for charge transport.
Tuning Adsorption Energy
The N-doping has a specific chemical benefit regarding lithium polysulfides.
It tunes the adsorption energy of the material, making it more effective at trapping polysulfides, which is a critical performance metric in lithium-sulfur battery applications.
Critical Process Considerations
Atmosphere Purity
While nitrogen is protective, the purity of the gas flow is paramount.
Any introduction of oxygen or moisture due to leaks in the tube furnace can compromise the pyrolysis and degrade the heterojunction quality.
Temperature Uniformity
The effectiveness of the annealing depends heavily on the furnace's ability to maintain a consistent temperature profile.
Fluctuations during the pyrolysis phase can lead to incomplete conversion of precursors or uneven N-doping across the CNT network.
Making the Right Choice for Your Goal
To maximize the effectiveness of this synthesis step, align your process controls with your specific material objectives:
- If your primary focus is Structural Stability: Prioritize the integrity of the inert atmosphere to ensure the complete and contamination-free conversion of metal precursors into ZnS/CeO2.
- If your primary focus is Electrochemical Performance: Optimize the annealing duration and temperature to maximize the efficiency of N-doping within the CNTs for better conductivity and adsorption.
This heat treatment is the defining step that transforms raw precursors into a functional, high-performance composite material.
Summary Table:
| Process Function | Key Benefit | Impact on Material |
|---|---|---|
| Controlled Pyrolysis | Stable Heterojunction Formation | Converts metal precursors into ZnS/CeO2 structures |
| Protective Barrier | Prevention of Oxidation | Protects samples from contamination and air-based reactions |
| Nitrogen-Doping | Chemical Modification of CNTs | Enhances electrical conductivity and electronic properties |
| Adsorption Tuning | Polysulfide Trapping | Improves performance in lithium-sulfur battery applications |
Elevate Your Material Research with KINTEK Precision
Achieving the perfect ZnS/CeO2 heterojunction requires rigorous control over atmosphere purity and temperature uniformity. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to facilitate advanced pyrolysis and N-doping processes.
Backed by expert R&D and precision manufacturing, our high-temperature furnaces are fully customizable to meet your unique laboratory needs. Whether you are optimizing electrochemical performance or structural stability, our equipment ensures the consistent environment necessary for high-performance composite synthesis.
Ready to upgrade your lab's capabilities? Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Yulin Luo, Qi-Hui Wu. Carbon Nanotubes-Doped Metal Oxides and Metal Sulfides Heterostructure Achieves 3D Morphology Deposition of Li2S and Stable Long-Cycle Lithium–Sulfur Batteries. DOI: 10.3390/inorganics13060181
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is it necessary to use a high-purity argon gas protective atmosphere? Ensure Precision in Brazing Filler Melting
- Why is a reducing atmosphere important? Prevent Oxidation for Superior Material Processing
- What are the applications of inert atmosphere furnaces? Essential for Metal Processing, Electronics, and Additive Manufacturing
- How does the box type annealing atmosphere furnace improve production efficiency? Boost Throughput and Cut Costs
- Why is furnace atmosphere control important in heat treatment processes? Ensure Precision and Quality in Material Processing
- What are metallizing furnaces used for? Bond Metal to Ceramic for Advanced Electronics
- What are the dual functions of the inner cover in a bell-type annealing furnace? Heat Transfer and Protective Sealing
- How is helium utilized in atmosphere furnaces? Unlock Purity and Rapid Cooling for Superior Results



















