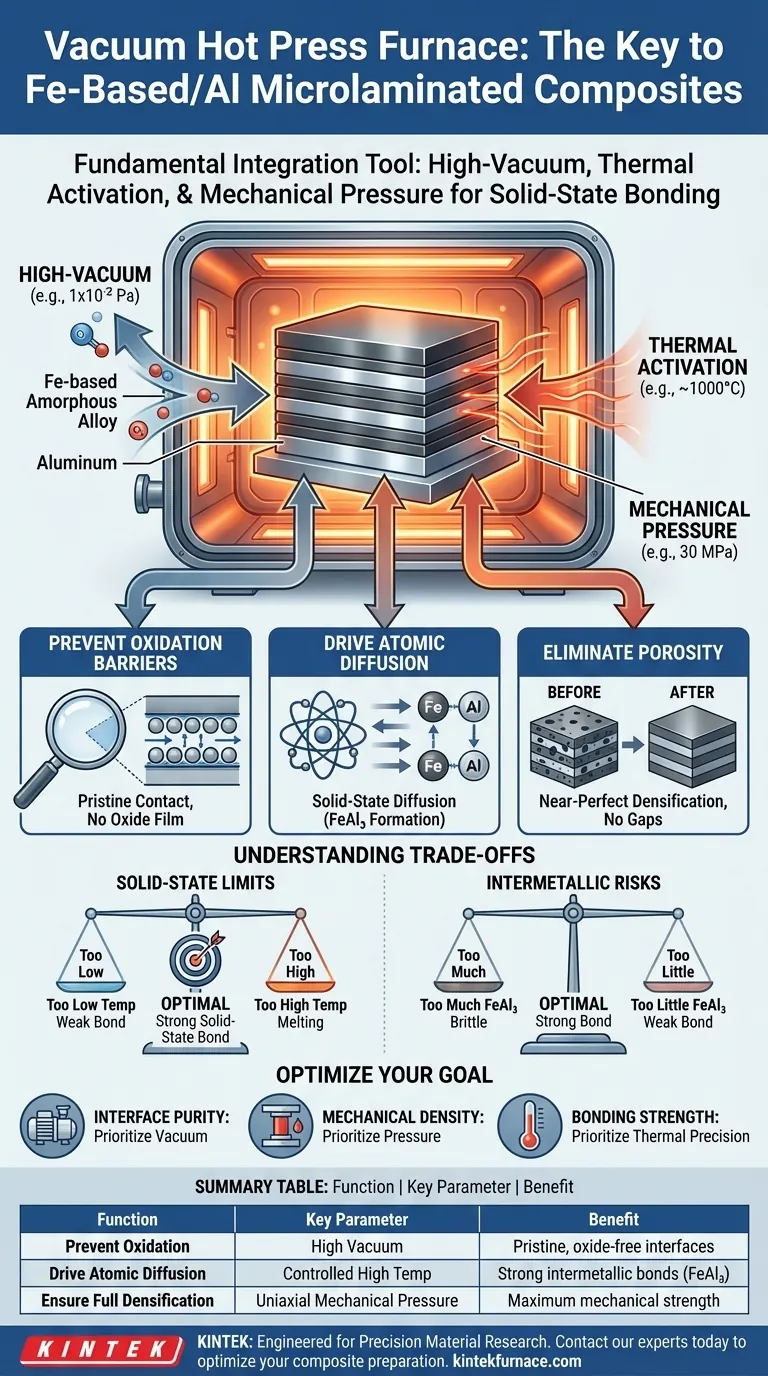
The vacuum hot press furnace serves as the fundamental integration tool for preparing Fe-based amorphous alloy/aluminum microlaminated composites. It provides a controlled environment that simultaneously applies thermal activation and mechanical pressure within a high-vacuum chamber. This combination is essential to achieve solid-state metallurgical bonding, prevent the rapid oxidation of aluminum, and ensure the complete densification of the composite layers.
The core value of this equipment lies in its ability to overcome the natural barriers to bonding between Iron and Aluminum. By removing oxygen and applying pressure-assisted heat, it forces atoms to diffuse across layers, creating a unified, high-strength structural material without melting the components.

The Mechanics of Composite Formation
Preventing Oxidation Barriers
The presence of oxygen is the primary enemy of Aluminum bonding. Aluminum naturally forms a tough oxide film that acts as a barrier to atomic interaction. The furnace creates a high-vacuum environment (e.g., $1 \times 10^{-2}$ Pa) to prevent these oxide films from forming. This ensures a pristine contact interface, maintaining open channels for elemental diffusion between the amorphous alloy and aluminum layers.
Driving Atomic Diffusion
Merely pressing layers together is insufficient for structural integrity; the materials must bond at an atomic level. The furnace uses thermal activation (e.g., temperatures around 1000°C, depending on the specific alloy) to stimulate the movement of iron and aluminum atoms. This promotes solid-state diffusion, leading to the formation of controlled intermetallic compounds like $FeAl_3$, which are responsible for the high-strength metallurgical bond.
Eliminating Porosity
Gaps and voids between layers severely compromise the mechanical strength of a composite. The furnace applies uniaxial mechanical pressure (e.g., 30 MPa) to the material stack. This pressure forces plastic deformation and rearrangement of the materials, effectively squeezing out gas pockets and closing interlayer pores to achieve near-perfect densification.
Understanding the Trade-offs
Solid-State Limits vs. Bonding Quality
While high temperature aids diffusion, the process must remain a solid-state reaction. If the temperature is too low, diffusion is sluggish, and the bond will be weak. However, if the temperature is too high, you risk melting the aluminum or altering the properties of the Fe-based amorphous alloy. The furnace allows for precise thermal control to hit this narrow window.
Intermetallic Formation Risks
The formation of compounds like $FeAl_3$ is necessary for bonding, but an over-abundance can be detrimental. Thick layers of intermetallics can introduce brittleness to the composite. The vacuum hot press process requires careful calibration of time and temperature to ensure enough diffusion for adhesion, but not so much that the interface becomes brittle.
Making the Right Choice for Your Goal
To optimize the preparation of your Fe-based/Aluminum composites, consider these operational priorities:
- If your primary focus is Interface Purity: Prioritize the vacuum capabilities of the furnace; a higher vacuum level is non-negotiable to strip away adsorbed moisture and prevent oxide barriers.
- If your primary focus is Mechanical Density: Focus on the hydraulic pressure capacity; higher pressure is required to facilitate plastic deformation and eliminate microscopic pores at the interface.
- If your primary focus is Bonding Strength: Concentrate on thermal precision; you must maintain temperatures that maximize atomic diffusion ($FeAl_3$ formation) without crossing into liquid-phase reactions.
Mastering the vacuum hot press parameters transforms incompatible distinct layers into a single, high-performance engineering material.
Summary Table:
| Function | Key Parameter | Benefit |
|---|---|---|
| Prevent Oxidation | High Vacuum (e.g., 1x10⁻² Pa) | Creates pristine, oxide-free bonding interfaces |
| Drive Atomic Diffusion | Controlled High Temperature (e.g., ~1000°C) | Promotes formation of strong intermetallic bonds (e.g., FeAl₃) |
| Ensure Full Densification | Uniaxial Mechanical Pressure (e.g., 30 MPa) | Eliminates porosity for maximum mechanical strength |
Ready to transform your material research with precision-controlled solid-state bonding?
Our vacuum hot press furnaces are engineered to deliver the exact combination of high vacuum, precise temperature control, and uniform pressure required for creating high-performance microlaminated composites like Fe-based amorphous alloy/aluminum. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs.
Contact our experts today to discuss how a KINTEK vacuum hot press furnace can be optimized for your specific composite preparation goals.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What role does a vacuum hot pressing furnace play in TiBw/TA15 synthesis? Enhance In-Situ Composite Performance
- What are the advantages of SPS for BCZY712 electrolytes? Achieve 98% Density and Superior Proton Conductivity
- How does vacuum hot pressing compare to vacuum brazing and sintering? Choose the Right Process for Your Materials
- What is the function of a Boron Nitride (BN) coating in hot press sintering? Protect Your Fluoride Ceramics Now
- How are hot press furnaces involved in semiconductor manufacturing? Essential for Wafer Bonding in 3D ICs
- How does vacuum hot pressing or pressureless sintering equipment facilitate GdEuZrO preparation? Achieve High Density
- What are the key advantages of hot pressing in terms of material quality? Achieve Superior Density and Purity for High-Performance Materials
- What is vacuum hot pressing (VHP) and what materials is it suitable for? Unlock High-Density Material Solutions



















