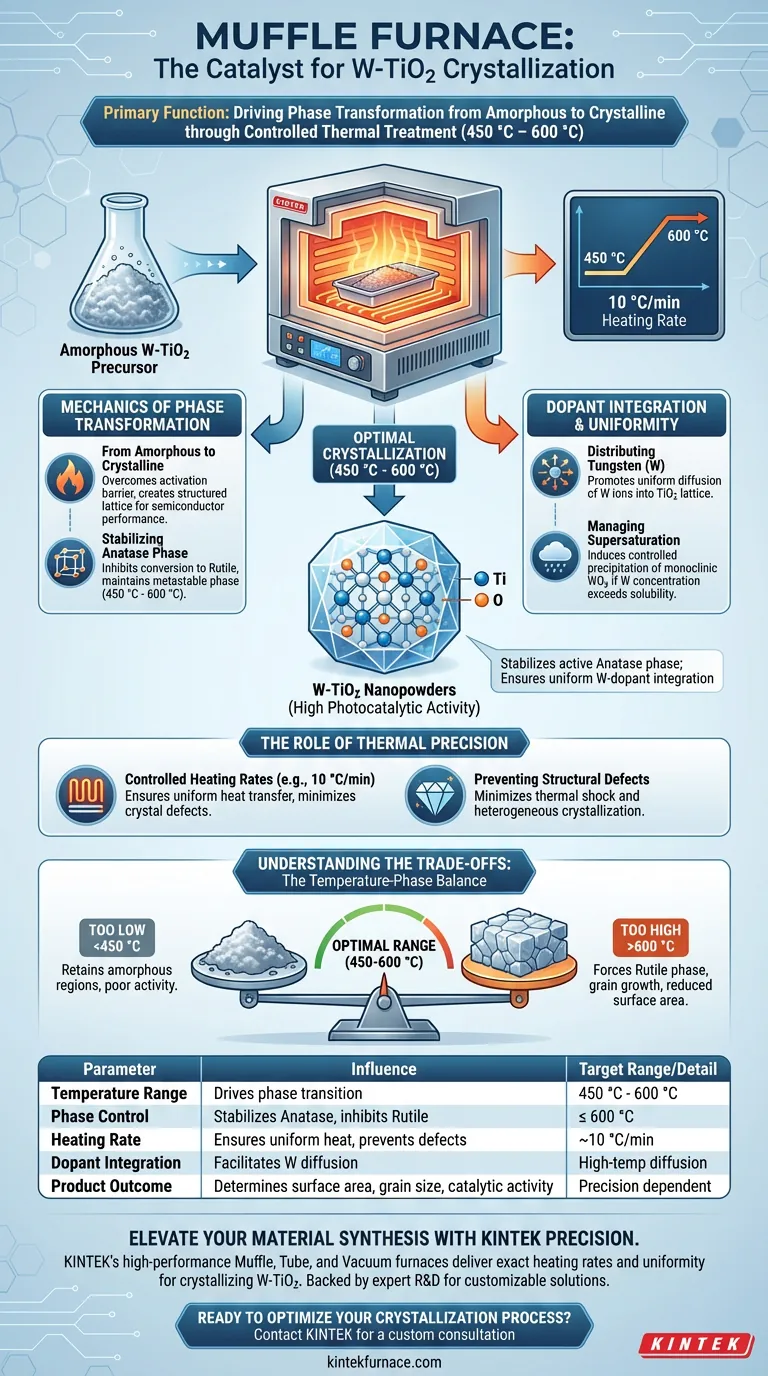
The primary function of a muffle furnace in this context is to drive the phase transformation from amorphous precursors to crystalline structures through controlled thermal treatment. specifically, it creates a stable high-temperature environment (typically 450 °C to 600 °C) that crystallizes W-doped Titanium Dioxide (W-TiO2) nanopowders. This process is critical for establishing the material's final structural properties and catalytic performance.
The muffle furnace acts as a precision instrument for lattice engineering, stabilizing the highly active anatase phase while ensuring the uniform integration of Tungsten dopants into the Titanium Dioxide structure.
The Mechanics of Phase Transformation
Transitioning from Amorphous to Crystalline
Before treatment in a muffle furnace, the W-TiO2 precursor exists as an amorphous powder with no defined long-range order. The furnace provides the necessary thermal energy to overcome the activation barrier for crystallization. This converts the disordered arrangement of atoms into a structured, crystalline lattice essential for semiconductor performance.
Stabilizing the Anatase Phase
For W-TiO2, the goal is often to retain the metastable anatase phase, which is generally more photocatalytically active than the thermodynamic equilibrium phase (rutile). By maintaining temperatures between 450 °C and 600 °C, the muffle furnace facilitates the formation of anatase while effectively inhibiting the conversion to the rutile phase.
Dopant Integration and Uniformity
Distributing Tungsten (W) within the Lattice
The high-temperature environment promotes the diffusion of atoms, allowing Tungsten ions to integrate uniformly into the Titanium Dioxide (TiO2) lattice. This substitution is vital for modifying the electronic band structure of the material.
Managing Supersaturation
In scenarios where the Tungsten concentration exceeds the solubility limit of the TiO2 lattice, the muffle furnace plays a slightly different role. It induces the controlled precipitation of monoclinic WO3. This ensures that any excess dopant forms a secondary phase in a predictable manner rather than clustering randomly as defects.
The Role of Thermal Precision
Controlled Heating Rates
A muffle furnace allows for programmable heating rates, such as 10 °C/min. This gradual ramp-up ensures uniform heat transfer throughout the powder sample.
Preventing Structural Defects
Rapid or uneven heating can lead to thermal shock or heterogeneous crystallization. By controlling the rate of temperature increase, the furnace minimizes crystal defects and ensures the final nanopowders possess high crystallinity and structural integrity.
Understanding the Trade-offs
The Temperature-Phase Balance
Operating the furnace requires a delicate balance. If the temperature is too low (below 450 °C), the material may retain amorphous regions or organic residues from the synthesis process, resulting in poor activity.
The Risk of Overheating
Conversely, exceeding the optimal temperature range (e.g., going well above 600 °C) can force the material into the rutile phase. While stable, rutile often exhibits lower photocatalytic efficiency compared to anatase for many applications. Furthermore, excessive heat can lead to grain growth (sintering), which reduces the specific surface area of the nanopowders.
Making the Right Choice for Your Goal
To optimize your W-TiO2 synthesis, you must align your furnace parameters with your specific material requirements.
- If your primary focus is maximum photocatalytic activity: Target the 450 °C – 500 °C range to maximize the surface area and ensure the preservation of the pure anatase phase.
- If your primary focus is dopant activation: Ensure your dwell time is sufficient to allow for the complete diffusion of Tungsten into the lattice, but monitor strictly for the onset of rutile transformation.
- If your primary focus is composite formation (TiO2/WO3): Use the higher end of the temperature spectrum to encourage the controlled precipitation of crystalline WO3 if you are working with supersaturated mixtures.
Success in synthesizing W-TiO2 relies not just on reaching a high temperature, but on the precise control of the thermal profile to dictate the atomic arrangement of the final crystal.
Summary Table:
| Parameter | Influence on W-TiO2 Crystallization | Target Range/Detail |
|---|---|---|
| Temperature Range | Drives phase transition from amorphous to crystalline | 450 °C - 600 °C |
| Phase Control | Stabilizes active Anatase phase; inhibits Rutile transition | ≤ 600 °C |
| Heating Rate | Ensures uniform heat transfer and prevents defects | ~10 °C/min |
| Dopant Integration | Facilitates Tungsten (W) diffusion into the TiO2 lattice | High-temp diffusion |
| Product Outcome | Determines surface area, grain size, and catalytic activity | Precision dependent |
Elevate Your Material Synthesis with KINTEK Precision
Precise lattice engineering requires uncompromising thermal control. KINTEK’s high-performance Muffle, Tube, and Vacuum furnaces are designed to deliver the exact heating rates and temperature uniformity essential for crystallizing W-TiO2 nanopowders with high photocatalytic activity.
Backed by expert R&D and world-class manufacturing, we provide customizable solutions—including CVD systems and rotary furnaces—tailored to your unique lab requirements. Ensure the structural integrity of your semiconductors today.
Ready to optimize your crystallization process?
Contact KINTEK for a custom consultation
Visual Guide
References
- Khley Cheng, Andreï Kanaev. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. DOI: 10.3390/nano14090765
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a high-temperature laboratory furnace play in BaTiO3? Master Dislocation Injection & Plasticity
- What safety features are important in a muffle furnace? Ensure Lab Safety with Advanced Protection Systems
- What is the difference between a retort and a muffle furnace? Choose the Right Atmosphere-Controlled Furnace
- What is the difference between a muffle furnace and a normal furnace? Choose the Right Tool for Purity vs. Speed
- What are the advantages of using a box type resistance furnace? Achieve Precision Heating for Your Lab
- Why is a vacuum muffle furnace used to perform proximate analysis? Precise Biomass Evaluation & Reactivity Analysis
- What is a muffle furnace and what is its primary purpose? Discover Precision Heating for Pure Results
- What role does a high-temperature muffle furnace play in KNN-based ceramic powder pre-sintering? Key Synthesis Insights



















