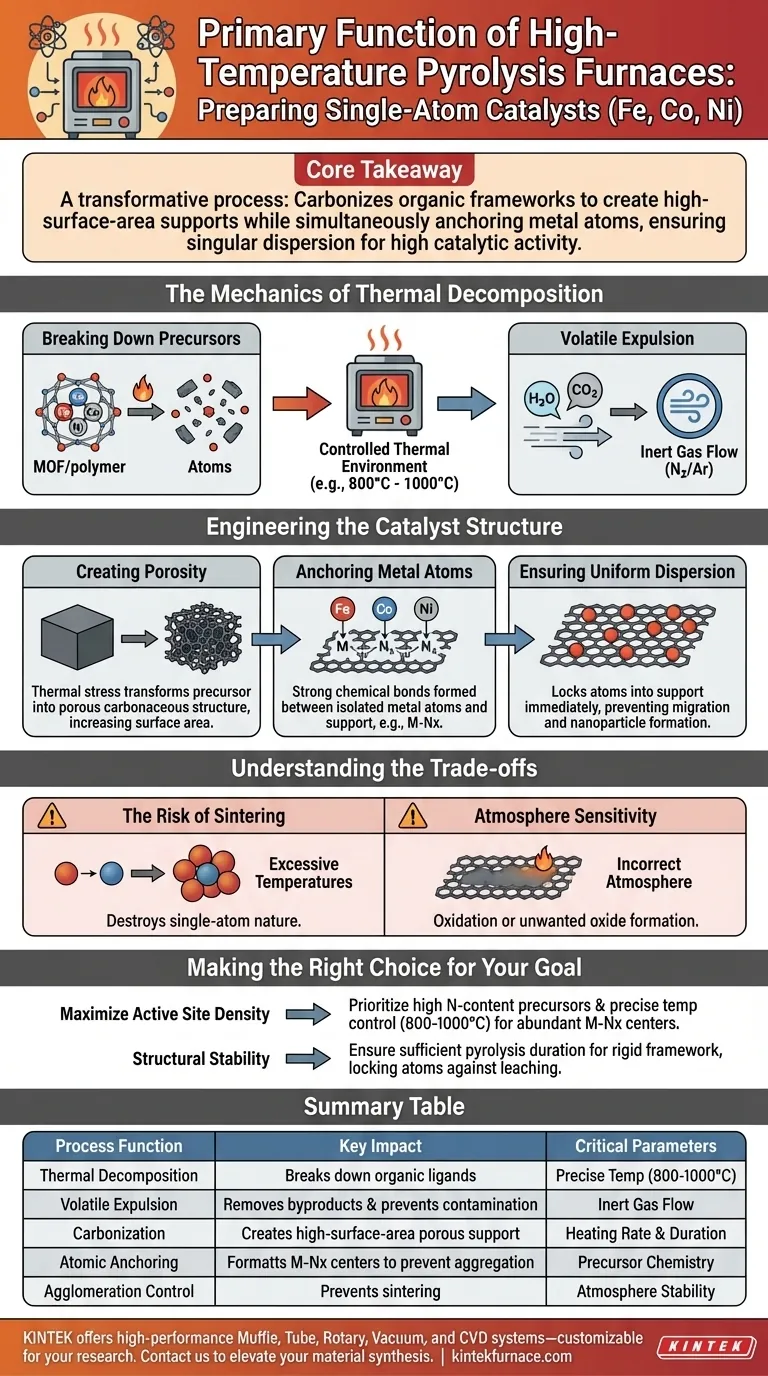
The primary function of a high-temperature pyrolysis furnace in this context is to provide a controlled thermal environment that facilitates the decomposition of organic ligands within metal precursors, such as Metal-Organic Frameworks (MOFs) or polymers. This thermal treatment is the critical step that converts raw chemical ingredients into a structured, functional catalyst.
Core Takeaway Pyrolysis is not merely about heating; it is a transformative process that carbonizes organic frameworks to create high-surface-area supports while simultaneously anchoring metal atoms. This prevents metal aggregation, ensuring the singular dispersion of Fe, Co, or Ni atoms necessary for high catalytic activity.

The Mechanics of Thermal Decomposition
Breaking Down Precursors
The furnace creates the necessary conditions to break down complex organic ligands found in precursors like MOFs or metal complexes.
This decomposition is the first step in releasing the metal atoms from their initial chemical bonds, preparing them for re-coordination.
Volatile Expulsion
As the organic material decomposes, volatile byproducts must be removed from the material matrix.
The furnace, often utilizing an inert gas flow (such as Nitrogen), ensures these decomposition products are efficiently expelled, preventing contamination of the final catalyst structure.
Engineering the Catalyst Structure
Creating Porosity
The thermal stress induced by the furnace transforms the precursor material into a porous carbonaceous structure.
This process significantly increases the surface area, which is vital for exposing the maximum number of active sites to reactants during future catalytic applications.
Anchoring Metal Atoms
Perhaps the most critical function is the creation of strong chemical bonds between the isolated metal atoms and the support material.
By controlling the temperature (often between 800°C and 1000°C), the furnace facilitates the coordination of metal atoms with elements like Nitrogen within the carbon support (forming Fe-Nx centers, for example).
Ensuring Uniform Dispersion
Proper pyrolysis prevents the metal atoms from migrating and clumping together.
By locking the atoms into the support structure immediately upon decomposition, the furnace ensures the metals remain atomically dispersed rather than aggregating into nanoparticles.
Understanding the Trade-offs
The Risk of Sintering
While high heat is necessary for carbonization and anchoring, excessive temperatures can lead to "sintering."
Sintering occurs when thermal energy overcomes the anchoring forces, causing single atoms to migrate and merge into larger metallic clusters, effectively destroying the "single-atom" nature of the catalyst.
Atmosphere Sensitivity
The success of the pyrolysis depends heavily on the atmospheric environment maintained within the furnace.
An incorrect atmosphere (e.g., lack of inert gas protection) can lead to the oxidation of the carbon support or the unwanted formation of metal oxides rather than the desired metal-nitrogen-carbon coordination.
Making the Right Choice for Your Goal
To optimize the synthesis of Fe, Co, and Ni single-atom catalysts, align your thermal treatment strategy with your specific structural requirements.
- If your primary focus is maximizing active site density: Prioritize precursors with high nitrogen content and precise temperature control (around 800-1000°C) to facilitate the formation of abundant metal-nitrogen (M-Nx) coordination centers.
- If your primary focus is structural stability: Ensure the pyrolysis duration is sufficient to fully carbonize the support, creating a rigid framework that locks metal atoms in place against leaching or movement.
A precisely tuned pyrolysis process is the difference between a high-performance single-atom catalyst and a generic aggregate of metal particles.
Summary Table:
| Process Function | Key Impact on Catalyst Structure | Critical Parameters |
|---|---|---|
| Thermal Decomposition | Breaks down organic ligands and precursors | Precise Temperature (800°C - 1000°C) |
| Volatile Expulsion | Removes byproducts and prevents contamination | Inert Gas Flow (N2/Argon) |
| Carbonization | Creates high-surface-area porous carbon support | Heating Rate & Duration |
| Atomic Anchoring | Formats M-Nx centers to prevent aggregation | Precursor Chemistry & Environment |
| Agglomeration Control | Prevents sintering of atoms into nanoparticles | Atmosphere Stability |
Precision is paramount when engineering single-atom catalysts. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous thermal demands of your research. Whether you are optimizing Fe-Nx centers or scaling carbonization, our furnaces provide the temperature uniformity and atmospheric control needed to prevent sintering and ensure atomic dispersion. Contact KINTEK today to discover how our lab high-temperature solutions can elevate your material synthesis.
Visual Guide
References
- Yuquan Yang, Jinlong Zheng. Preparation of Fe, Co, Ni-based single atom catalysts and the progress of their application in electrocatalysis. DOI: 10.20517/microstructures.2024.65
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the main disadvantages of rotary furnaces? Key Limitations for Material Processing
- What fuel does a rotary furnace use? Optimize Your Thermal Process with the Right Energy Source
- What technology has advanced as an alternative to traditional rotary kilns? Discover Electromagnetic Heating for Efficiency
- What factors should be considered when evaluating the suitability of a rotary tube furnace for a process? Optimize Your Thermal Processing
- What key principles make rotary kilns efficient for high-temperature processing? Unlock Optimal Thermal Processing
- How is heat transferred to the furnace tubes in a rotary tube furnace? Master Uniform Heating for Your Materials
- What are the main structural components of a rotary furnace? Explore Key Parts for Efficient Material Processing
- How are rotary furnaces used in coating preparation? Master Bulk Powder Processing for Durable Coatings



















