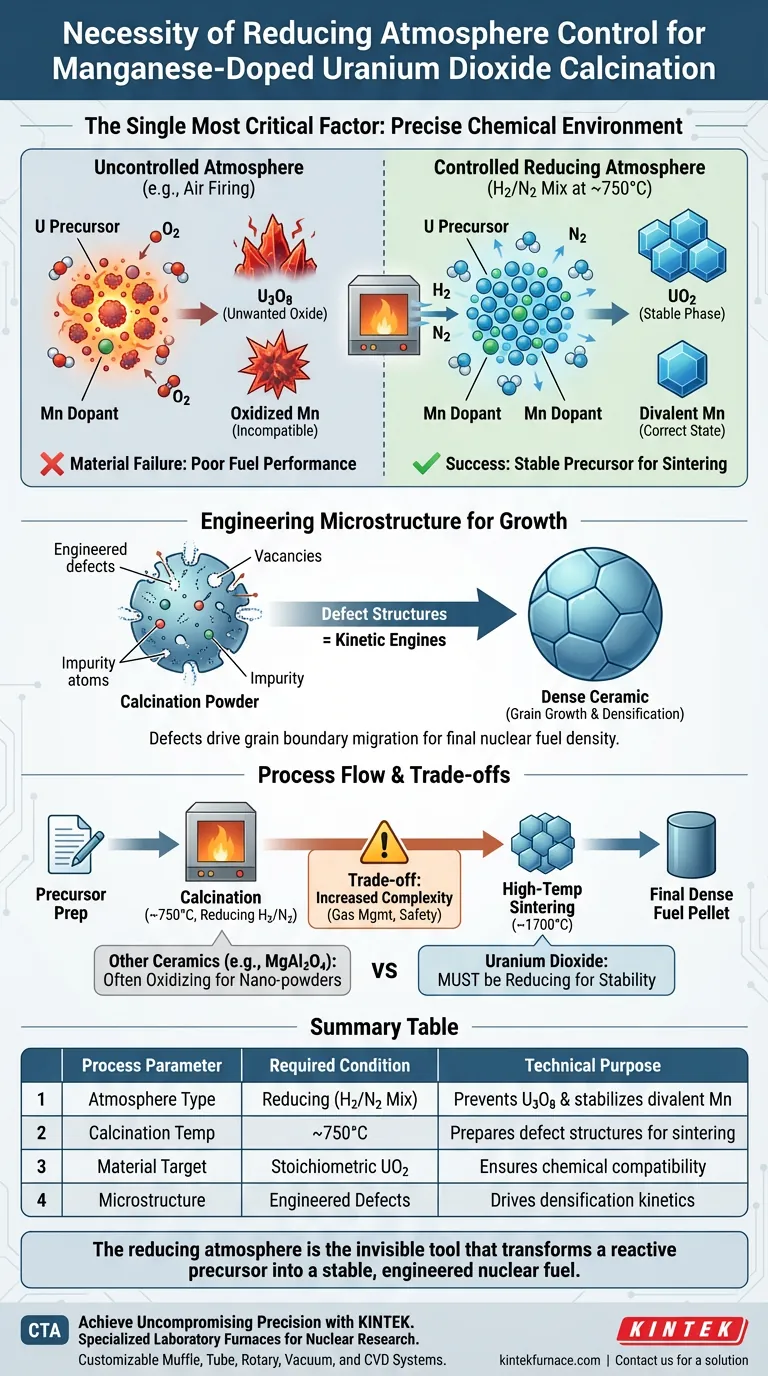
Precise control of the chemical environment is the single most critical factor in successfully processing manganese-doped uranium dioxide. The necessity of using a laboratory furnace with a reducing atmosphere control system lies in its ability to prevent the uncontrolled oxidation of uranium while stabilizing the manganese dopant. By maintaining a specific gas mixture (such as hydrogen-nitrogen) at temperatures around 750°C, the system ensures the precursor converts into a stable uranium dioxide phase rather than unwanted higher oxides.
Core Takeaway: Atmosphere control is not merely a protective measure; it is an active processing variable that dictates the material's stoichiometry. Without a reducing environment, you cannot maintain the divalent state of manganese or create the specific defect structures required to drive grain growth and densification in the final nuclear fuel.
Preserving Critical Valence States
The primary function of the reducing atmosphere is to lock specific elements into their required chemical states. In standard air firing, these elements would naturally drift toward stable, yet undesirable, oxidized forms.
Preventing Uranium Oxidation
Uranium is highly reactive with oxygen. Without a reducing atmosphere, the uranium in the precursor will oxidize into higher valence states (such as U3O8) during heat treatment.
To produce effective nuclear fuel, the material must remain as Uranium Dioxide (UO2). The reducing atmosphere (typically a Hydrogen-Nitrogen mix) actively strips excess oxygen, preventing the formation of higher oxides that would degrade the fuel's performance.
Stabilizing the Manganese Dopant
The effectiveness of manganese as a dopant depends entirely on its valence state. The process specifically requires manganese to exist in a divalent state.
If processed in an uncontrolled atmosphere, manganese may oxidize to a state that does not integrate correctly into the uranium lattice. The reducing environment protects the manganese, ensuring it remains chemically compatible for subsequent doping mechanisms.
Engineering Defect Structures for Growth
Beyond simple chemical protection, the atmosphere control system allows you to engineer the microstructure of the material at the atomic level.
Promoting Grain Growth
The ultimate goal of adding manganese is to influence how the grains of the ceramic grow. The reducing atmosphere facilitates the formation of defect structures within the crystal lattice.
These defects are the kinetic engines that drive grain boundary migration. They allow the material to evolve from a powder into a dense ceramic with the specific grain size required for safety standards.
Setting the Stage for Sintering
Calcination at 750°C is a preparatory step for high-temperature sintering (which occurs around 1700°C). If the calcination atmosphere is incorrect, the powder will lack the necessary characteristics for densification later.
Properly calcined powders allow manganese atoms to diffuse into and substitute within the uranium lattice during the final sintering phase, leading to a denser, more uniform fuel pellet.
Understanding the Trade-offs
While atmosphere control is necessary, it introduces complexity that must be managed. It is useful to understand how this differs from other material processes to appreciate the strict requirements of UO2.
Atmosphere Sensitivity vs. Other Materials
Not all ceramics require reduction. For example, materials like MgAl2O4 are often calcined in pure oxygen to inhibit volume diffusion and promote surface diffusion for nano-powders.
However, applying this logic to Uranium Dioxide would be catastrophic. The "trade-off" here is that you cannot rely on standard oxidative mechanisms to refine particle size; you must rely strictly on chemical reduction to achieve stability.
The Cost of Precision
Atmosphere furnaces are more complex than standard air muffle furnaces. They require gas management systems and safety protocols for handling hydrogen.
However, this complexity is unavoidable. Attempting to bypass this equipment requirement results in a fundamental failure to produce the correct chemical phase, rendering the material useless for nuclear applications.
Making the Right Choice for Your Goal
When selecting equipment or designing your process flow, consider your specific analytical targets.
- If your primary focus is Phase Purity: Ensure your furnace can maintain a stable Hydrogen-Nitrogen flow at 750°C to guarantee the Uranium remains as UO2 and Manganese remains divalent.
- If your primary focus is Grain Kinetics Research: You must verify that your atmosphere control is precise enough to generate consistent defect structures, as these defects are the variable that will dictate your grain growth results during post-sintering analysis.
The reducing atmosphere is the invisible tool that transforms a reactive precursor into a stable, engineered nuclear fuel.
Summary Table:
| Process Parameter | Required Condition | Technical Purpose |
|---|---|---|
| Atmosphere Type | Reducing (H2/N2 Mix) | Prevents U3O8 formation & stabilizes divalent Manganese |
| Calcination Temp | ~750°C | Prepares defect structures for high-temp sintering |
| Material Target | Stoichiometric UO2 | Ensures chemical compatibility and fuel performance |
| Microstructure | Engineered Defects | Drives grain boundary migration for densification |
Achieve Uncompromising Precision in Your Nuclear Research
Maintaining the delicate stoichiometry of manganese-doped uranium dioxide requires more than just heat—it requires absolute atmospheric control.
Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of nuclear material processing. Whether you need to maintain a divalent manganese state or promote specific grain kinetics, our laboratory high-temp furnaces are fully customizable to meet your unique research needs.
Ready to elevate your material synthesis?
Contact KINTEK today for a customized furnace solution
Visual Guide
References
- H. R. W. Smith, Claire L. Corkhill. Fabrication, defect chemistry and microstructure of Mn-doped UO2. DOI: 10.1038/s41598-023-50676-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- In which fields is the inert atmosphere principle commonly applied? Discover Key Uses in Heat Treatment, Food, and More
- How does inert atmosphere heat treating benefit aluminum? Prevent Oxide Buildup for Superior Results
- What are the characteristics of atmosphere furnaces? Unlock Precise Heat Treatment for Superior Materials
- How does the box type annealing atmosphere furnace improve production efficiency? Boost Throughput and Cut Costs
- How does an industrial box resistance furnace activate TiO2/ZSM-5 catalysts? Expert Calcination Insights
- Why is controlled atmosphere capability important in an atmosphere furnace? Unlock Precise Material Processing
- Why is atmosphere control critical for heat treatment quality? Unlock Precision and Durability
- What factors should be considered when choosing a controlled atmosphere furnace? Ensure Optimal Performance for Your Materials



















