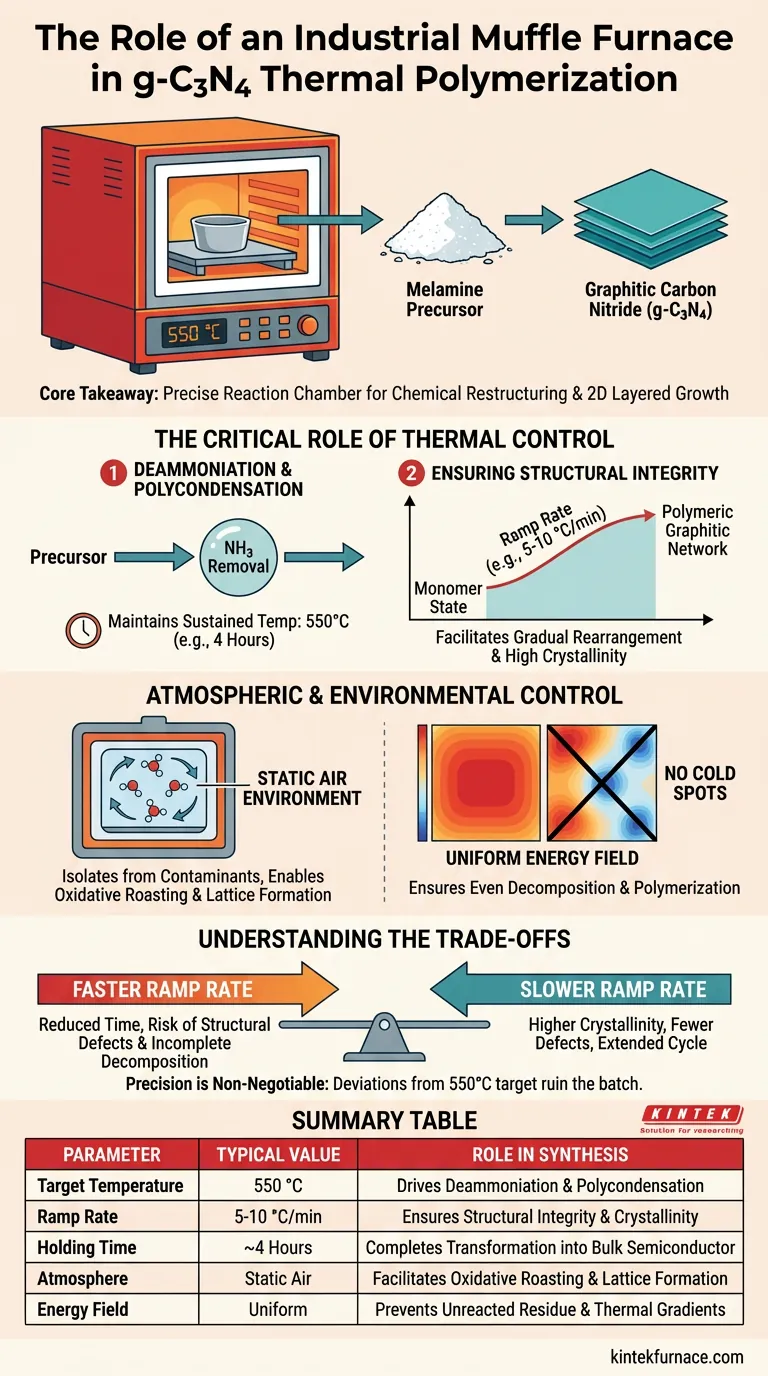
The primary function of an industrial muffle furnace in the synthesis of graphitic carbon nitride (g-C3N4) is to provide a strictly controlled, high-temperature static air environment—typically at 550 °C—required to drive thermal polymerization. By regulating the heating rate and holding time, the furnace ensures that precursors like melamine undergo complete deammoniation and polycondensation, transforming monomers into a stable, layered bulk semiconductor material.
Core Takeaway The muffle furnace is not merely a heat source but a precise reaction chamber that facilitates the chemical restructuring of melamine into graphitic carbon nitride. Its ability to maintain a stable 550 °C temperature under an air atmosphere is the deciding factor in creating the necessary two-dimensional, layered structure required for high-performance applications.

The Critical Role of Thermal Control
The synthesis of g-C3N4 is a thermal polycondensation process. The muffle furnace manages the energy input required to break specific chemical bonds in the precursor and form new, stable linkages.
Driving Deammoniation and Polycondensation
The fundamental chemical reaction occurring inside the furnace is the removal of ammonia (deammoniation) followed by the linking of molecules (polycondensation).
To achieve this, the furnace must maintain a sustained temperature, generally around 550 °C, for a specific duration (often 4 hours). This thermal energy forces the melamine precursor to transition from a monomeric state into a polymeric graphitic network.
Ensuring Structural Integrity via Heating Rates
The "ramp rate"—how fast the furnace heats up—is as important as the final temperature.
A controlled heating rate (e.g., 5 °C/min to 10 °C/min) allows the precursor molecules to rearrange gradually. This prevents the rapid release of gases that could destroy the material's structure, ensuring the formation of a highly crystalline and structurally regular framework.
Facilitating Layered Growth
The ultimate goal of this process is to create "bulk" g-C3N4 with a specific 2D layered structure.
The muffle furnace ensures the material grows securely, often within a crucible or on a carrier. This layered bulk structure is the essential starting point for producing ultrathin nanosheets in subsequent processing steps.
Atmospheric and Environmental Control
Beyond temperature, the muffle furnace dictates the chemical atmosphere surrounding the sample.
The Necessity of a Static Air Environment
Unlike processes requiring vacuum or inert gas, g-C3N4 synthesis typically utilizes a static air atmosphere.
The muffle furnace isolates the sample from external contaminants while allowing the necessary oxidative roasting conditions. This environment supports the dissociation of intergrowths and the proper formation of the carbon nitride lattice.
Uniform Energy Field
Industrial muffle furnaces are designed to minimize thermal gradients.
By providing a uniform energy field, the furnace ensures that the precursor material decomposes and polymerizes evenly throughout the batch. This prevents "cold spots" that would result in incomplete reactions or unreacted melamine residue.
Understanding the Trade-offs
While the muffle furnace is the standard tool for this synthesis, operators must navigate specific process limitations to ensure quality.
Ramp Rate vs. Process Efficiency
There is a direct trade-off between the speed of the heating ramp and the quality of the crystallization.
A faster ramp rate reduces total processing time but increases the risk of structural defects and incomplete decomposition. A slower rate (e.g., 5 °C/min) yields higher crystallinity and fewer defects but significantly extends the production cycle.
Temperature Sensitivity
Precision is non-negotiable.
DEviating significantly from the optimal 550 °C target can ruin the batch. Excessive heat may cause the material to decompose entirely or oxidize into unwanted byproducts, while insufficient heat will fail to trigger the necessary polymerization, leaving behind raw precursor.
Making the Right Choice for Your Goal
When configuring an industrial muffle furnace for g-C3N4 synthesis, your specific parameters should align with your end-product requirements.
- If your primary focus is high crystallinity: utilize a conservative heating ramp (approx. 5 °C/min) and ensure a full 4-hour holding time to minimize structural defects.
- If your primary focus is process consistency: ensure your furnace is calibrated to eliminate thermal gradients, guaranteeing that the static air atmosphere reaches the precursor uniformly across the entire chamber.
Success in synthesizing graphitic carbon nitride relies not just on reaching 550 °C, but on the disciplined control of the thermal journey to get there.
Summary Table:
| Parameter | Typical Value | Role in g-C3N4 Synthesis |
|---|---|---|
| Target Temperature | 550 °C | Drives deammoniation and polycondensation |
| Ramp Rate | 5-10 °C/min | Ensures structural integrity and crystallinity |
| Holding Time | ~4 Hours | Completes transformation into bulk semiconductor |
| Atmosphere | Static Air | Facilitates oxidative roasting and lattice formation |
| Energy Field | Uniform | Prevents unreacted residue and thermal gradients |
Maximize Your Material Performance with KINTEK
High-quality graphitic carbon nitride requires more than just heat; it demands the absolute precision and thermal uniformity found in KINTEK’s advanced industrial furnaces. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored specifically for your laboratory and production needs.
Whether you are refining thermal polymerization or scaling up 2D material synthesis, our customizable solutions ensure your process is repeatable and efficient. Contact KINTEK today to consult with our specialists and find the perfect high-temperature furnace for your unique application.
Visual Guide
References
- Junping Zhang, Hongzhi An. Novel electrochemical platform based on C3N4-graphene composite for the detection of neuron-specific enolase as a biomarker for lung cancer. DOI: 10.1038/s41598-024-56784-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the operating principle of a muffle furnace? Unlock Pure, Controlled Heating for Your Lab
- What is the function of the muffle chamber in the furnace? Ensure Purity and Uniform Heating
- How does a muffle furnace contribute to the synthesis of carbon-supported NiO nanocomposites? Master Thermal Precision
- How does high-temp sintering affect shale ceramics? Enhance Strength with Electric Chamber Furnaces
- What are the primary applications of a muffle furnace? Essential for Analysis and Heat Treatment
- How do muffle furnaces contribute to drug testing in pharmaceuticals? Ensure Purity and Compliance with Precision
- What is the function of a high-temperature muffle furnace in LATP pre-calcination? Essential Solid-Phase Reactions
- What is the technical necessity of using a laboratory muffle furnace for Zinc Oxide? Master ZnO Synthesis Precision



















