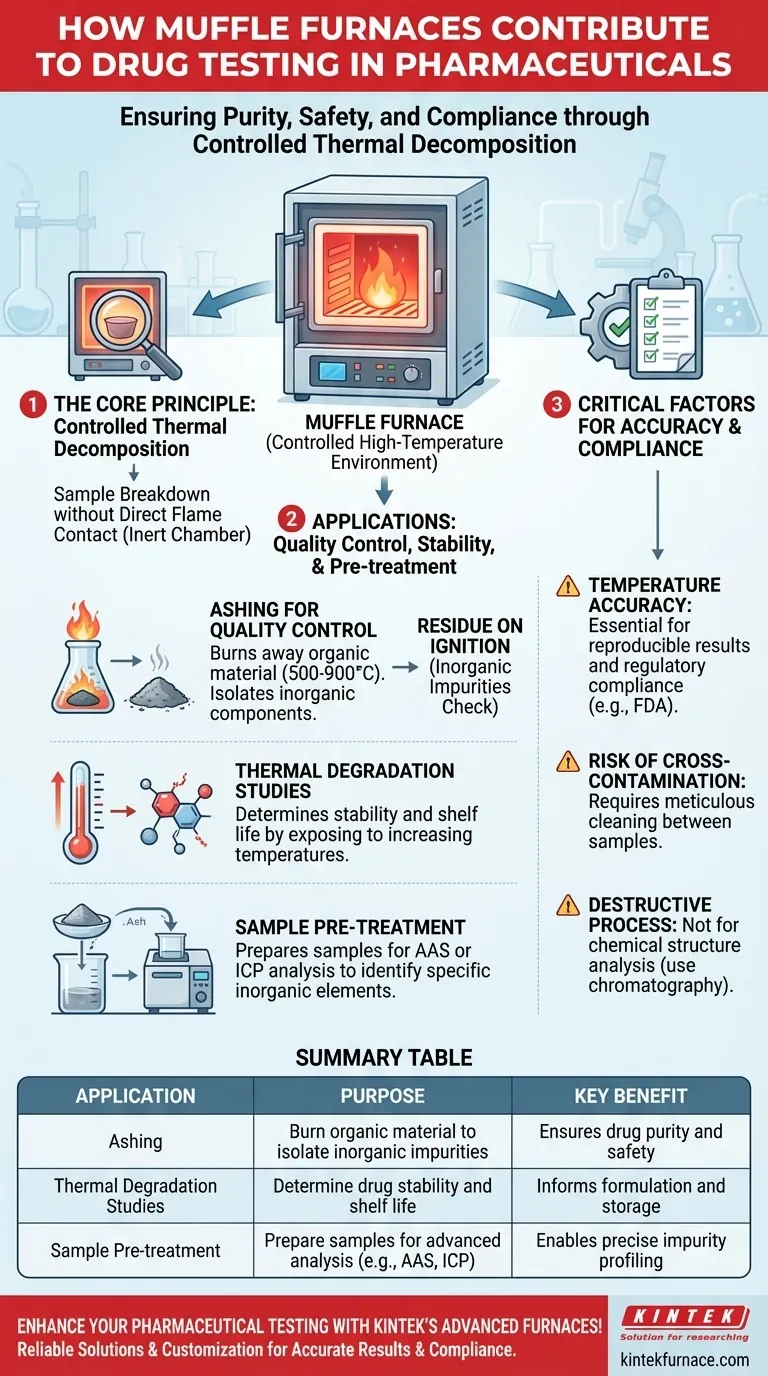
In essence, a muffle furnace contributes to pharmaceutical drug testing by using a precisely controlled, high-temperature environment to break down drug samples. This process, often called ashing or thermal degradation, is essential for isolating and quantifying inorganic components, assessing drug stability, and preparing samples for further analysis, thereby ensuring the drug’s purity, safety, and compliance with regulatory standards.
A muffle furnace's primary role in drug testing is not analysis itself, but controlled deconstruction. By systematically breaking down a sample with extreme heat, it allows scientists to verify its composition and stability, which is a fundamental requirement for ensuring patient safety.

The Core Principle: Controlled Thermal Decomposition
A muffle furnace is fundamentally a high-temperature oven that heats its contents without direct contact from flames or heating elements. This is achieved by placing the sample inside a "muffle"—an enclosed, inert chamber.
Ashing for Quality Control
The most common application in drug testing is ashing. This process involves heating a sample to a very high temperature (typically 500-900°C) to completely burn away all organic material, including the active pharmaceutical ingredient (API) and any organic excipients.
What remains is a small amount of ash, which consists entirely of the inorganic components.
Determining Inorganic Impurities
This resulting ash is weighed and analyzed to perform a critical quality control check. The amount and composition of the ash can reveal the presence of inorganic impurities, such as residual heavy metal catalysts from the manufacturing process or other contaminants.
This test, often referred to as "residue on ignition," is a standard procedure required by pharmacopeias to ensure the purity and safety of the final drug product.
Applications in Stability and Formulation
Beyond simple ashing, muffle furnaces are indispensable tools for research and development, helping to define a drug's fundamental characteristics.
Thermal Degradation Studies
Scientists use muffle furnaces to conduct thermal degradation studies. By exposing a drug to systematically increasing temperatures, they can determine the point at which it begins to break down.
This data is vital for understanding a drug's intrinsic stability, which helps in defining its shelf life and recommended storage conditions (e.g., "store below 25°C").
Sample Pre-treatment for Further Analysis
Often, the muffle furnace is the first step in a multi-stage analytical process. The ash produced from a sample is frequently dissolved and then analyzed using more sensitive techniques like atomic absorption spectroscopy (AAS) or inductively coupled plasma (ICP).
These subsequent methods can precisely identify and quantify the specific inorganic elements present in the ash, providing a detailed impurity profile.
Understanding the Critical Factors
Using a muffle furnace correctly is paramount, as errors can invalidate test results and compromise quality assurance.
The Importance of Temperature Accuracy
For test results to be reproducible and reliable, the temperature inside the furnace must be extremely accurate and uniform. Inconsistent heating can lead to incomplete combustion or variable degradation rates.
This accuracy is not just a technical detail; it is a requirement for regulatory compliance, as agencies like the FDA demand proof that testing methods are validated and controlled.
The Risk of Cross-Contamination
The muffle chamber must be meticulously cleaned between samples. Any residue from a previous test can contaminate the current sample, leading to a false reading of its inorganic content.
Not a Universal Tool
It is crucial to recognize that muffle furnaces are destructive. The process destroys the organic API and is therefore unsuitable for analyzing the drug's chemical structure or identifying organic impurities. Other methods, like chromatography, are used for that purpose.
Making the Right Choice for Your Goal
To leverage a muffle furnace effectively, align its use with your specific analytical objective.
- If your primary focus is routine quality control: Use the furnace for ashing (residue on ignition) to quantify total inorganic content and ensure it falls within specified limits.
- If your primary focus is research and development: Employ the furnace for thermal degradation studies to establish the drug's stability profile and inform formulation decisions.
- If your primary focus is regulatory compliance: Ensure your furnace is properly calibrated and the testing method is validated to produce defensible data for submissions.
Ultimately, the muffle furnace serves as a powerful gatekeeper in pharmaceutical science, ensuring the foundational purity and stability of medicines we rely on.
Summary Table:
| Application | Purpose | Key Benefit |
|---|---|---|
| Ashing | Burn organic material to isolate inorganic impurities | Ensures drug purity and safety |
| Thermal Degradation Studies | Determine drug stability and shelf life | Informs formulation and storage |
| Sample Pre-treatment | Prepare samples for advanced analysis (e.g., AAS, ICP) | Enables precise impurity profiling |
Enhance your pharmaceutical testing with KINTEK's advanced high-temperature furnaces! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, helping you achieve accurate results, regulatory compliance, and improved efficiency. Contact us today to discuss how our furnaces can support your drug testing goals!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What types of analyses can be performed using a muffle furnace in coal analysis? Unlock Key Coal Quality Insights
- What process conditions must a muffle furnace satisfy for CoNiCrAlY oxidation? Ensure Precise High-Temp Stability
- What are the primary functions of industrial high-temperature muffle or tube furnaces in the sintering of PCEC?
- Why is temperature uniformity important in a muffle furnace? Ensure Precise and Reliable Results
- Why are crucible furnaces ideal for small-scale operations? Maximize Efficiency in Your Workshop
- What is the role of muffle furnaces in incineration processes? Precision Ashing for Accurate Material Analysis
- What are the key components of a muffle furnace as shown in its diagram? Discover Its Core Architecture
- Why is it necessary to control the heating rate of a muffle furnace during calcination? Optimize Bioactive Glass Quality



















