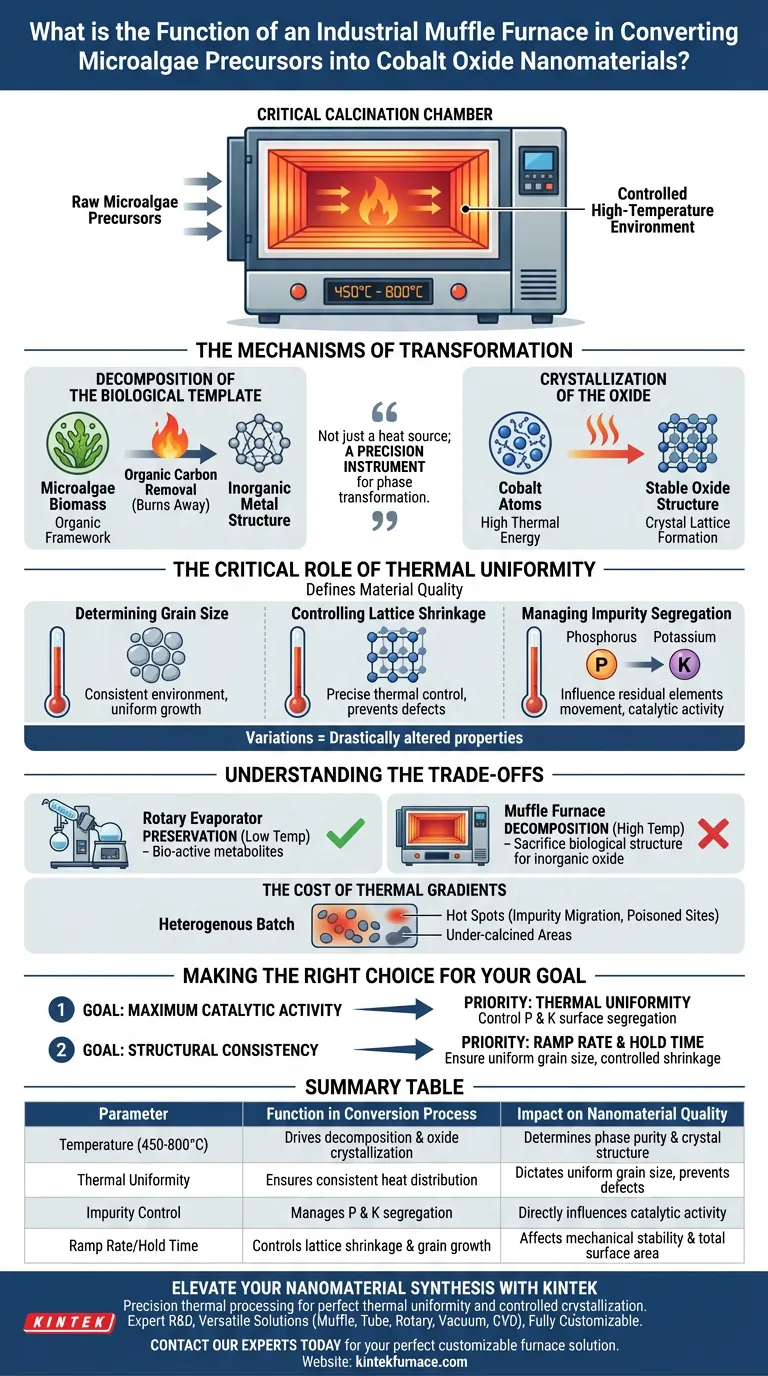
The industrial muffle furnace serves as the critical calcination chamber where raw microalgae precursors are transformed into functional cobalt oxide nanomaterials. By maintaining a controlled high-temperature environment—typically between 450°C and 800°C—it drives the chemical decomposition of the biological template while simultaneously crystallizing the remaining cobalt into its oxide form.
The muffle furnace is not merely a heat source; it is a precision instrument for phase transformation. Its ability to maintain a uniform thermal field directly dictates the crystal structure and surface chemistry of the final material, distinguishing high-performance catalysts from substandard byproducts.
The Mechanisms of Transformation
The conversion process within the furnace relies on two simultaneous physical-chemical events.
Decomposition of the Biological Template
The primary function of the furnace is to remove the organic framework.
As the temperature rises, the microalgae biomass—which acted as a carrier or template for the cobalt—burns away. This decomposition removes organic carbon, leaving behind the inorganic metal structure.
Crystallization of the Oxide
Simultaneously, the high thermal energy facilitates the formation of the crystal lattice.
The heat forces the cobalt atoms to arrange themselves into a stable oxide structure. The specific temperature chosen (e.g., 450°C vs. 800°C) determines how fully this crystallization occurs.
The Critical Role of Thermal Uniformity
While temperature induces the reaction, the uniformity of the thermal field defines the quality of the result. Variations in heat distribution within the chamber can drastically alter the material's properties.
Determining Grain Size
A consistent thermal environment ensures that crystal grains grow evenly.
Uniform heating prevents the formation of disparate grain sizes, which is essential for predicting the material's mechanical stability and surface area.
Controlling Lattice Shrinkage
As the material crystallizes, the atomic lattice contracts or "shrinks."
precise thermal control is required to manage this shrinkage. Inconsistent heating can lead to structural defects or internal stresses within the nanomaterial.
Managing Impurity Segregation
The furnace's heat profile influences the movement of residual elements derived from the microalgae, specifically phosphorus (P) and potassium (K).
Thermal uniformity dictates whether these elements remain trapped within the bulk material or segregate to the surface. This surface segregation is a deciding factor in the final catalytic activity of the cobalt oxide.
Understanding the Trade-offs
It is vital to distinguish the role of the furnace from the preparation steps that precede it.
Preservation vs. Decomposition
While equipment like a rotary evaporator is used earlier to preserve bio-active metabolites at low temperatures, the muffle furnace is designed for controlled decomposition.
You cannot maintain bio-reductive activity in the furnace; its purpose is to sacrifice the biological structure to create the inorganic oxide.
The Cost of Thermal Gradients
If the furnace fails to maintain a uniform thermal field, the resulting batch of nanomaterials will be heterogeneous.
This leads to "hot spots" where impurities like phosphorus migrate excessively to the surface, potentially poisoning the catalytic sites, while other areas of the batch remain under-calcined.
Making the Right Choice for Your Goal
To maximize the potential of your cobalt oxide nanomaterials, you must tune the furnace parameters to your specific end-use requirements.
- If your primary focus is Maximum Catalytic Activity: Prioritize the uniformity of the thermal field to precisely control the surface segregation of phosphorus and potassium, as these surface impurities drive the reaction.
- If your primary focus is Structural Consistency: Focus on the ramp rate and hold time at specific temperatures (450–800 °C) to ensure uniform grain size and controlled lattice shrinkage across the entire sample.
The quality of your final nanomaterial is less about the precursor you start with and more about the precision of the thermal environment that transforms it.
Summary Table:
| Parameter | Function in Conversion Process | Impact on Nanomaterial Quality |
|---|---|---|
| Temperature (450-800°C) | Drives decomposition of bio-templates and oxide crystallization. | Determines phase purity and crystal structure. |
| Thermal Uniformity | Ensures consistent heat distribution across the sample. | Dictates uniform grain size and prevents structural defects. |
| Impurity Control | Manages the segregation of residual elements like P and K. | Directly influences the catalytic activity of the surface. |
| Ramp Rate/Hold Time | Controls the speed of lattice shrinkage and grain growth. | Affects mechanical stability and total surface area. |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision thermal processing is the bridge between raw microalgae precursors and high-performance catalysts. KINTEK provides the advanced technology required to achieve perfect thermal uniformity and controlled crystallization for your most demanding lab and industrial applications.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our systems are engineered for precise phase transformations.
- Versatile Solutions: From Muffle, Tube, and Rotary Furnaces to Vacuum and CVD systems, we cover all high-temp needs.
- Fully Customizable: Tailor your thermal environment to manage specific lattice shrinkage and impurity segregation requirements.
Ready to optimize your material quality? Contact our technical experts today to find the perfect customizable furnace solution for your unique research needs.
Visual Guide
References
- Agnieszka Sidorowicz, Günther Rupprechter. Microalgae-derived Co<sub>3</sub>O<sub>4</sub> nanomaterials for catalytic CO oxidation. DOI: 10.1039/d4ra00343h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does the heating process work in a muffle furnace? Discover Clean, Uniform Heating for Your Lab
- How does a laboratory muffle furnace facilitate the activation of ZMQ-1 zeolite? Unlock 28-Ring Pore Channels
- How does the built-in venting system in a muffle furnace improve performance? Boost Durability and Safety in Your Lab
- What role does a Muffle Furnace play in biomass pellet torrefaction? Optimize Your High-Temperature Processing
- How should materials be selected for use in a Muffle furnace? Optimize Your High-Temperature Processes
- What specific thermal conditions must a high-temperature muffle furnace provide for BiOI to BiVO4 conversion?
- What is the working principle of the box furnace's door mechanism? Ensure Safety and Efficiency in Your Lab
- What are some applications of Muffle Furnaces? Unlock Clean, Controlled Heat for Your Lab



















