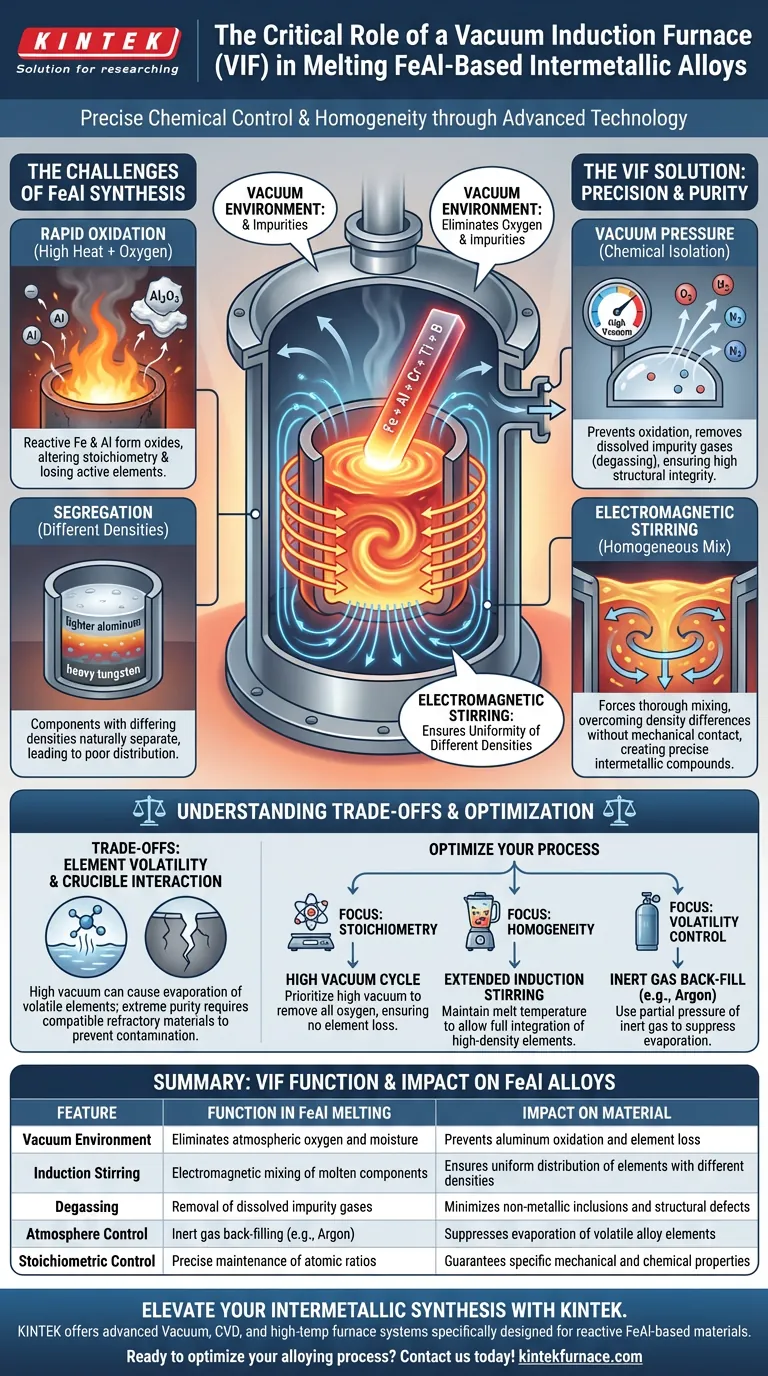
A vacuum induction furnace acts as the primary defense mechanism against chemical degradation during the synthesis of FeAl-based intermetallic alloys. By utilizing electromagnetic induction within a vacuum or controlled atmosphere, this apparatus melts iron and aluminum while rigorously preventing the oxidation of these highly active elements. This process allows for the precise chemical control necessary to create complex, high-purity formulations like Fe40Al5Cr0.2TiB.
Core Takeaway Processing FeAl-based alloys requires more than just high heat; it requires strict chemical isolation. The vacuum induction furnace solves the two biggest challenges in intermetallic synthesis: it utilizes vacuum pressure to eliminate oxygen and impurities, and employs electromagnetic stirring to ensure a uniform atomic distribution of elements with vastly different densities.

The Critical Role of Environment Control
Preventing Rapid Oxidation
Iron aluminides (FeAl) contain high concentrations of aluminum, a chemically "active" element that oxidizes rapidly when exposed to oxygen at high temperatures.
The primary function of the vacuum induction furnace is to eliminate atmospheric oxygen. By melting in a vacuum, the system ensures that active elements are not lost to oxide formation, preserving the intended stoichiometry of the alloy.
Elimination of Volatile Impurities
Beyond simply blocking oxygen, the vacuum environment actively cleans the material.
The low-pressure environment facilitates the removal of impurity gases dissolved in the raw materials. This "degassing" process minimizes the formation of non-metallic inclusions, ensuring the final casting possesses the high structural integrity required for advanced applications.
Achieving Homogeneity Through Induction
The Mechanism of Electromagnetic Stirring
A distinct advantage of induction heating over other melting methods is the generation of electromagnetic forces within the molten metal.
Because Iron and Aluminum (and dopants like Tungsten or Chromium) have different densities, they naturally tend to separate or segregate. The induction field induces a stirring motion in the melt, forcing these components to mix thoroughly without the need for mechanical intervention.
Precise Composition Control
FeAl-based materials are intermetallics, meaning their properties rely on specific atomic ratios rather than a loose mixture of elements.
The combination of a protected environment (preventing element loss) and electromagnetic stirring (ensuring mixing) allows for exact control over chemical composition. This is critical when producing complex alloys such as Fe40Al5Cr0.2TiB, where even minor deviations in composition can drastically alter material performance.
Understanding the Trade-offs
While vacuum induction melting is superior for purity, it presents specific operational challenges that must be managed.
Volatility of Certain Elements
While the vacuum removes impurities, it can also cause the evaporation of desirable elements if they have high vapor pressures. Operators may need to introduce a partial pressure of inert gas (such as Argon at 500 mbar) to suppress evaporation while still protecting the melt from oxidation.
Crucible Interactions
The extreme purity required for these alloys means the interaction between the melt and the crucible is a potential contamination vector. While induction melting is cleaner than fossil-fuel firing, selecting the correct refractory material—or utilizing cold crucible techniques—is essential to prevent the vessel itself from contaminating the reactive FeAl alloy.
Making the Right Choice for Your Goal
To maximize the effectiveness of a vacuum induction furnace for FeAl alloys, tailor your process to your specific quality metrics:
- If your primary focus is Chemical Stoichiometry: Prioritize a high-vacuum pump-down cycle before melting to remove all oxygen, ensuring no active Aluminum is lost to oxidation.
- If your primary focus is Structural Homogeneity: Maintain the melt temperature under induction power for a set duration to allow electromagnetic stirring to fully integrate high-density elements like Tungsten.
- If your primary focus is Controlling Volatility: Utilize a back-fill of inert Argon gas during the melt stage to prevent the evaporation of specific alloy components.
Success in melting FeAl intermetallics depends not just on melting the metal, but on strictly controlling the atmosphere to freeze a precise chemical moment in time.
Summary Table:
| Feature | Function in FeAl Melting | Impact on Material |
|---|---|---|
| Vacuum Environment | Eliminates atmospheric oxygen and moisture | Prevents aluminum oxidation and element loss |
| Induction Stirring | Electromagnetic mixing of molten components | Ensures uniform distribution of elements with different densities |
| Degassing | Removal of dissolved impurity gases | Minimizes non-metallic inclusions and structural defects |
| Atmosphere Control | Inert gas back-filling (e.g., Argon) | Suppresses evaporation of volatile alloy elements |
| Stoichiometric Control | Precise maintenance of atomic ratios | Guarantees specific mechanical and chemical properties |
Elevate Your Intermetallic Synthesis with KINTEK
Precise chemical control is the difference between a high-performance alloy and a failed melt. Backed by expert R&D and manufacturing, KINTEK offers advanced Vacuum, CVD, and high-temp furnace systems specifically designed to handle the reactive nature of FeAl-based materials. Whether you need a standard Muffle or a customizable Vacuum Induction system, our solutions provide the strict isolation and homogeneity your research demands.
Ready to optimize your alloying process? Contact us today to find your custom lab solution!
Visual Guide
References
- J. Cebulski, Jadwiga Gabor. Structure and Corrosion Resistance of Fe40Al5Cr0.2TiB Alloy After Casting and After Homogenization Annealing. DOI: 10.3390/ma18020308
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What safety advantages do Vacuum Induction Melting Furnaces offer? Ensure Operator and Material Safety in High-Stakes Industries
- Why is a double-layer water-cooled stainless steel chamber used in equipment for preparing ultrafine magnesium powder via the evaporation-condensation method?
- What precious metals can be melted in induction furnaces? Efficient, Clean Melting for Gold, Silver, and Platinum Group Metals
- What factors should be considered when selecting an induction melting furnace for a business? Maximize Efficiency and ROI
- What are the key benefits of using an IGBT Vacuum Induction Melting Furnace? Achieve Superior Metal Purity and Control
- What are the key components of an IGBT-based induction heater circuit? Unlock Efficient High-Frequency Heating
- Why is repeated melting and flipping of alloy ingots necessary? Achieving Homogeneity in Mn–Ni–Fe–Si Alloys
- What is the role of a Vacuum Induction Melting (VIM) furnace in Cobalt-Rhenium alloy production? Ensure Pure Alloys



















