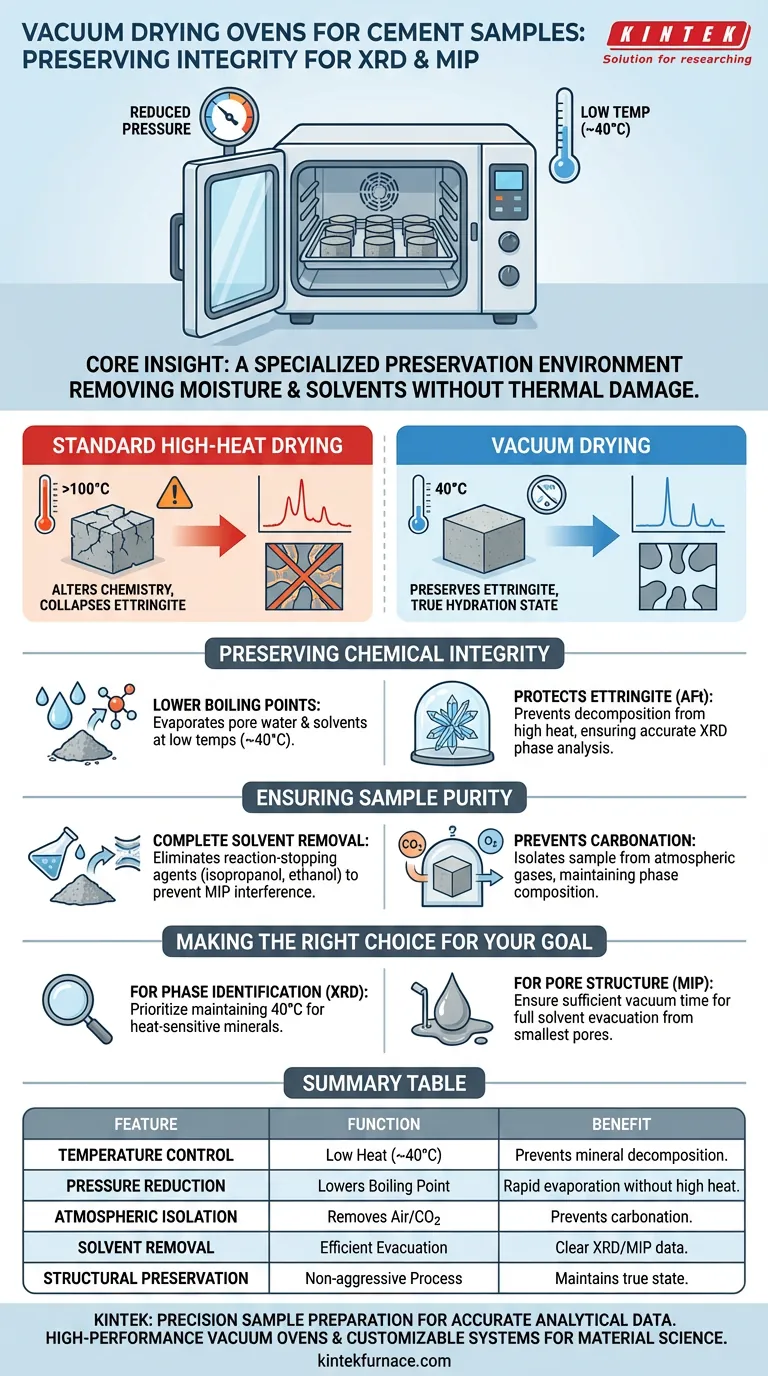
A vacuum drying oven functions as a specialized preservation environment that removes moisture and solvents from hardened cement samples without thermally damaging their delicate chemical structure.
By lowering the internal pressure, the oven allows volatile liquids—specifically the isopropanol or ethanol used to stop hydration—to evaporate at low temperatures (typically 40 °C). This ensures the sample remains chemically stable for sensitive analytical techniques like X-ray Diffraction (XRD) and Mercury Intrusion Porosimetry (MIP).
Core Insight Standard drying methods utilize high heat, which alters the very chemistry you are trying to measure. The vacuum drying oven solves this by using reduced pressure instead of excessive temperature to dry the sample, ensuring that heat-sensitive hydration products like ettringite remain intact for accurate analysis.

Preserving Chemical Integrity
The Physics of Low-Temperature Drying
The primary function of the vacuum oven is to lower the boiling point of liquids trapped within the cement matrix.
By creating a vacuum, the oven allows pore water and residual reaction-stopping solvents to evaporate vigorously at temperatures as low as 40 °C.
This bypasses the need for the high temperatures (often above 100 °C) required in standard ovens, which would otherwise be necessary to drive off moisture effectively.
Protecting Heat-Sensitive Minerals
Hardened cement paste contains delicate hydration products that are thermodynamically unstable at high temperatures.
Ettringite (AFt) is the most notable example; it decomposes easily when exposed to significant heat.
If a standard high-heat drying method were used, the ettringite crystalline structure would collapse, causing XRD analysis to falsely indicate its absence or reduced quantity.
Preventing Artificial Alterations
Vacuum drying ensures the microscopic test results reflect the true hydration state of the cement at the exact moment the reaction was stopped.
It eliminates the risk of "false negatives" in phase identification caused by thermal degradation during the preparation phase.
Ensuring Sample Purity
Removing Reaction-Stopping Solvents
Before drying, cement samples are typically immersed in solvents like isopropanol or ethanol to halt the hydration process.
The vacuum oven is critical for completely removing these residual solvents.
Failure to remove these organic fluids can interfere with pore structure analysis (MIP) or create background noise in spectral data.
Preventing Carbonation
Beyond temperature control, the vacuum environment isolates the sample from atmospheric gases.
This isolation protects the cement paste from carbonation, a reaction where atmospheric carbon dioxide reacts with calcium hydroxide in the sample.
Carbonation alters the phase composition and pore structure, which would skew the data in both XRD and MIP testing.
Understanding the Trade-offs
Balancing Time and Gentleness
While vacuum drying provides superior sample quality, it is generally a slower process than high-temperature oven drying.
The user must accept a longer preparation timeline to ensure data accuracy. Rushing this step by increasing the temperature defeats the purpose of using the vacuum.
Equipment Sensitivity
Vacuum drying requires precise maintenance of seals and pumps to ensure a stable negative pressure.
Inconsistent pressure can lead to incomplete drying, leaving residual solvent in the pores that will outgas during MIP testing, leading to erroneous porosity data.
Making the Right Choice for Your Goal
To maximize the accuracy of your cement analysis, apply the vacuum drying method based on your specific analytical targets:
- If your primary focus is Phase Identification (XRD): Prioritize maintaining the temperature strictly at 40 °C to preserve thermally sensitive crystals like ettringite.
- If your primary focus is Pore Structure (MIP): Ensure the vacuum cycle is sufficiently long to evacuate all solvents from the smallest pores, preventing blockage during mercury intrusion.
The vacuum drying oven is not merely a dryer; it is a stabilization tool that freezes the chemical state of your sample in time.
Summary Table:
| Feature | Vacuum Drying Function | Benefit for Cement Analysis |
|---|---|---|
| Temperature Control | Operates at low heat (~40°C) | Prevents decomposition of heat-sensitive minerals like ettringite. |
| Pressure Reduction | Lowers boiling point of liquids | Enables rapid evaporation of pore water and solvents without high heat. |
| Atmospheric Isolation | Removes air/CO2 from chamber | Prevents carbonation and chemical alteration of the cement matrix. |
| Solvent Removal | Efficiently evacuates isopropanol/ethanol | Ensures clear XRD spectral data and accurate MIP porosity readings. |
| Structural Preservation | Non-aggressive drying process | Maintains the true hydration state and pore structure for microscopy. |
Precision Sample Preparation Starts with KINTEK
Don't compromise your analytical data with improper drying techniques. KINTEK provides high-performance vacuum drying ovens and specialized lab furnaces designed to meet the rigorous demands of material science and cement research. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to protect your most delicate samples.
Ready to elevate your lab's accuracy? Contact our specialists today to find the perfect drying solution for your unique research needs.
Visual Guide
References
- Huanhuan Li, Zhenping Sun. Synergistic Improvement in Setting and Hardening Performance of OPC-CSA Binary Blended Cement: Combined Effect of Nano Calcium Carbonate and Aluminum Sulfate. DOI: 10.3390/app14052062
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the primary function of a vacuum oven for Mo-based catalyst precursors? Ensure Purity & Pore Integrity
- How does the perpendicular orientation of substrate holders benefit VTD? Maximize Efficiency and Thermal Control
- What is the function of a rotary high-pressure autoclave in the synthesis of SSZ-13 zeolites? | Enhance Crystallinity
- What is the specific function of hydrogen and helium in quartz glass melting? Optimize Your High-Temp Processes
- Why is precise temperature control in a vacuum drying oven critical for CoTe@Ti3C2 battery electrodes? Key Insights.
- What is the function of a high-pressure reactor in SHS? Optimize Tungsten Carbide Synthesis with Precision
- Why is annealing in a heat treatment furnace performed on graphite flake/copper composite samples before performance testing? Ensure Data Integrity for Precision Thermal Expansion Measurements
- What gas is used in a graphite furnace? A Guide to Argon vs. Nitrogen for Optimal Analysis



















