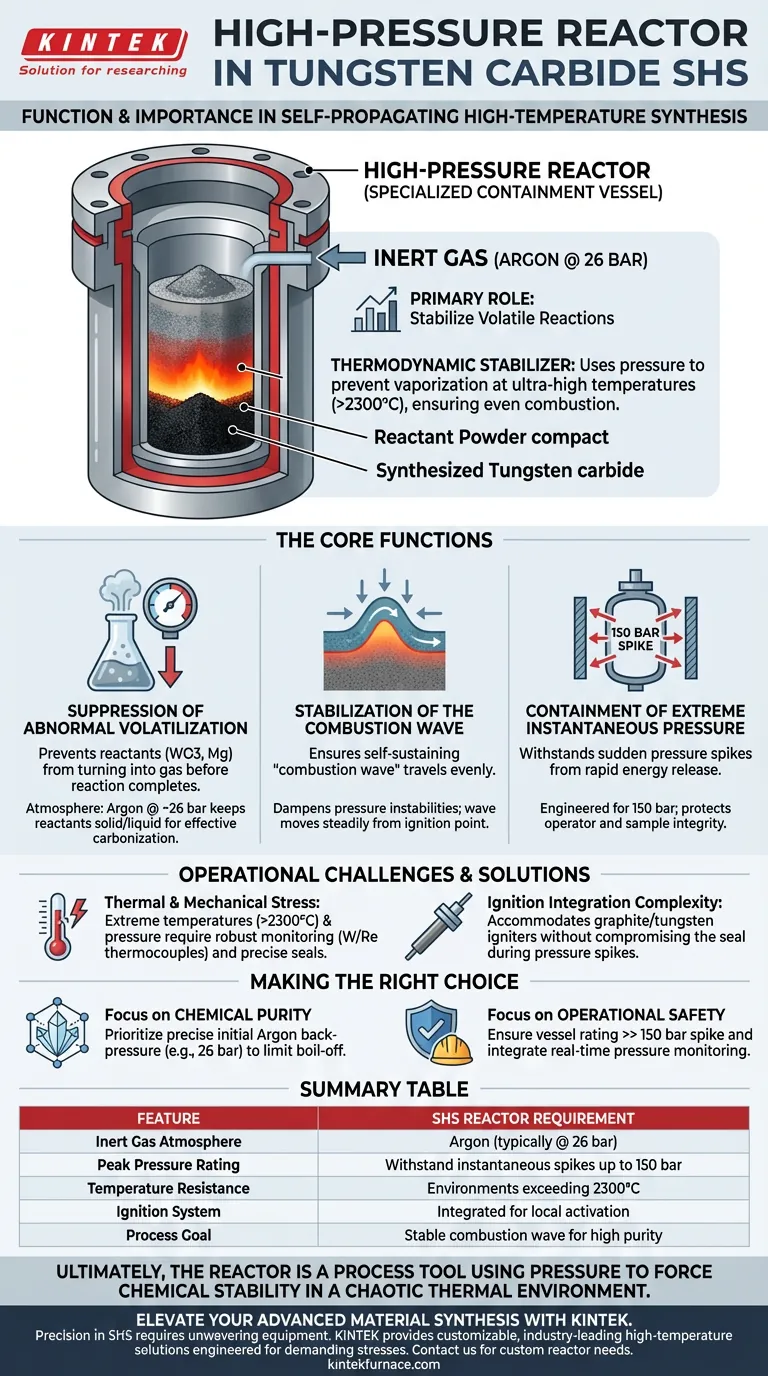
In the Self-propagating High-temperature Synthesis (SHS) of tungsten carbide, the high-pressure reactor functions as a specialized containment vessel designed to stabilize volatile chemical reactions under extreme conditions. Its primary role is to maintain a sealed environment filled with high-pressure inert gas (typically Argon at 26 bar) while withstanding instantaneous internal pressure spikes that can reach up to 150 bar.
The high-pressure reactor acts as a thermodynamic stabilizer, using inert gas pressure to prevent reactants from vaporizing at ultra-high temperatures, ensuring the combustion wave propagates evenly through the material to form pure tungsten carbide.

The Core Functions of the Reactor
Suppression of Abnormal Volatilization
The synthesis of tungsten carbide (specifically within the WO3-Mg-C system) generates immense heat, often exceeding 2300°C.
Without sufficient external pressure, reactants like Magnesium and Tungsten Trioxide would volatilize (turn into gas) before the reaction completes.
The reactor utilizes an atmosphere of Argon gas at approximately 26 bar to suppress this volatilization, keeping the reactants in the necessary solid or liquid phase for effective carbonization.
Stabilization of the Combustion Wave
For SHS to be successful, a self-sustaining "combustion wave" must travel through the powder compact.
Instabilities in pressure or gas expansion can disrupt this wave, leading to incomplete reactions or structural defects.
The reactor provides a controlled, sealed volume that dampens these instabilities, ensuring the reaction front moves steadily from the ignition point (triggered by graphite electrodes) through the entire reactant body.
Containment of Extreme Instantaneous Pressure
The exothermic nature of this reaction releases energy rapidly, creating sudden pressure spikes.
Standard reaction vessels would fail under the mechanical stress caused by the rapid expansion of gases and heat.
The high-pressure reactor is engineered to withstand instantaneous pressures of up to 150 bar, protecting both the operator and the integrity of the sample during the critical ignition and propagation phases.
Understanding the Operational Challenges
Thermal and Mechanical Stress Management
While the reactor contains the pressure, the internal components are subjected to extreme environments.
The system relies on robust monitoring, such as Tungsten-Rhenium thermocouples (W/Re-20), to track temperatures that can surpass the melting points of standard sensors.
Designing the vessel to maintain a perfect seal at 150 bar while accommodating 2300°C internal temperatures requires precise engineering and rigorous safety protocols.
Complexity of Ignition Integration
The reactor must accommodate external energy sources to start the process without compromising the pressure seal.
Graphite electrodes and tungsten wire igniters must be fed into the high-pressure zone to convert electrical energy into the thermal energy required for local ignition.
Failure in the feed-through seals during the pressure spike is a common failure mode that must be mitigated through design.
Making the Right Choice for Your Project
When utilizing a high-pressure reactor for SHS, your configuration depends on your specific outcome requirements.
- If your primary focus is Chemical Purity: Prioritize the precision of the initial Argon back-pressure (e.g., 26 bar) to strictly limit reactant boil-off and carbon loss.
- If your primary focus is Operational Safety: Ensure the vessel is rated significantly above the expected 150 bar spike and integrate real-time pressure monitoring to detect seal failures immediately.
Ultimately, the high-pressure reactor is not just a container, but a process tool that uses pressure to force chemical stability in a chaotic thermal environment.
Summary Table:
| Feature | SHS Reactor Function/Requirement |
|---|---|
| Inert Gas Atmosphere | Argon (typically @ 26 bar) to suppress reactant volatilization |
| Peak Pressure Rating | Must withstand instantaneous spikes up to 150 bar |
| Temperature Resistance | Operates in environments exceeding 2300°C |
| Ignition System | Integrated graphite electrodes/tungsten wire for local activation |
| Process Goal | Ensures stable combustion wave propagation for high purity |
Elevate Your Advanced Material Synthesis with KINTEK
Precision in Self-propagating High-temperature Synthesis (SHS) requires equipment that never wavers under pressure. KINTEK provides industry-leading, customizable high-temperature solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—engineered to withstand the most demanding thermal and mechanical stresses. Backed by expert R&D and world-class manufacturing, we help research labs and industrial manufacturers achieve superior material purity and process safety.
Ready to optimize your tungsten carbide production? Contact KINTEK today to discuss your custom reactor needs!
Visual Guide
References
- Carbon Loss and Control for WC Synthesis through a Self-propagating High-Temperature WO3-Mg-C System. DOI: 10.1007/s11665-025-10979-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What is the primary role of the Thermal Oxidation (TO) process in Ti-6Al-4V ELI alloy? Enhancing Hardness and Wear
- What is the purpose of performing high-temperature thermal treatment for BSnO thin films? Boost Device Sensitivity
- Why are acid washing and vacuum drying ovens required after carbon activation? Unlock Maximum Purity and Pore Access
- Why are high-precision constant temperature baths necessary? Unlock Accurate Fiber Optic Sensor Calibration
- What are the primary objectives of using a blast drying oven for In2O3/C nanofibers? Ensure Structural Integrity
- What is the function of a drying oven during the chemical activation of biochar? Optimize Your Porous Carbon Structure
- Why does the use of a forced-air drying oven often lead to increased particle size? Avoid Silica Agglomeration
- What is the necessity of a laboratory vacuum drying oven for photocatalytic powders? Protect Your Material Integrity



















