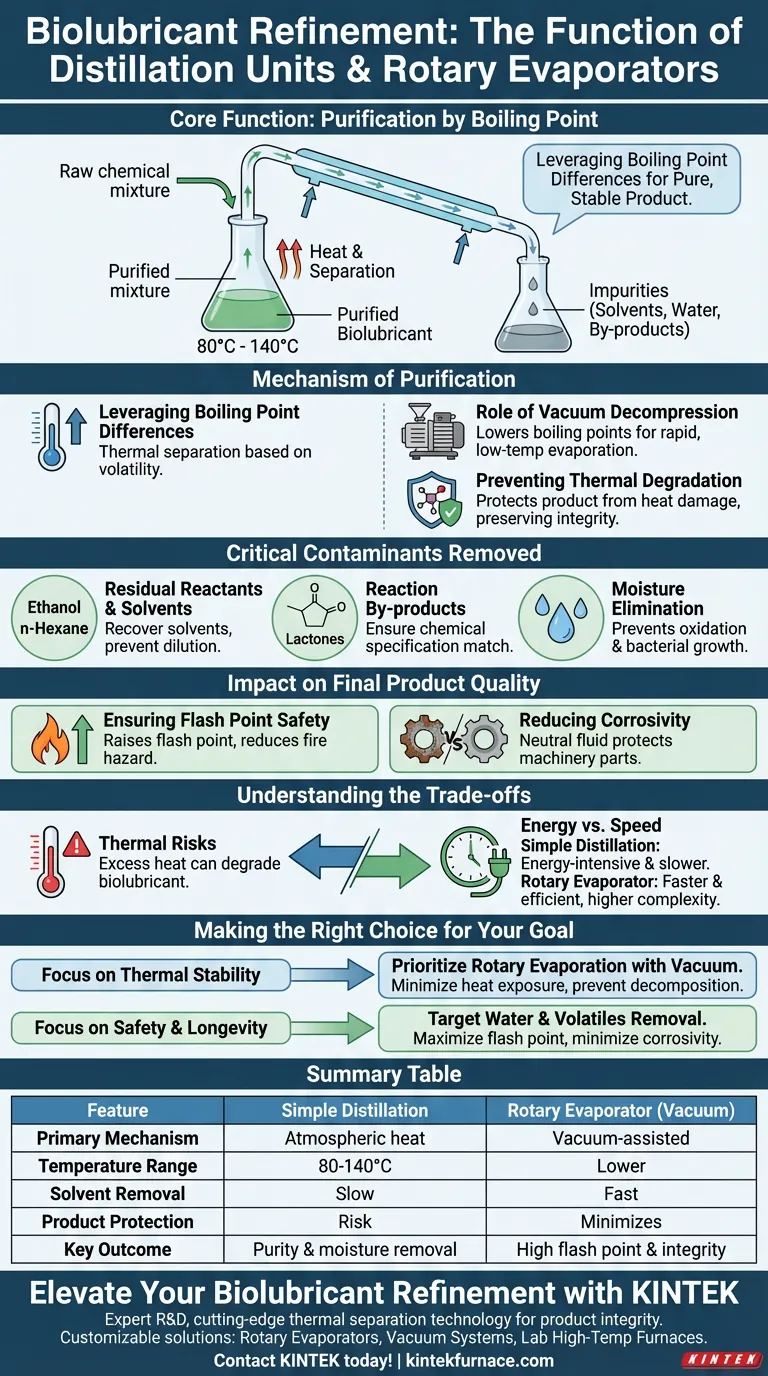
The primary function of a simple distillation unit or rotary evaporator during biolubricant refinement is to purify the product by leveraging differences in boiling points. By strictly controlling temperatures—typically between 80°C and 140°C—these units separate the desired biolubricant from residual ethanol, water, and reaction by-products.
The distillation process is the critical gateway between a raw chemical mixture and a functional lubricant. It ensures the final fluid is not only pure but also chemically stable, non-corrosive, and safe to operate at high temperatures.

The Mechanism of Purification
Leveraging Boiling Point Differences
The core principle involves heating the liquid mixture to specific temperatures where volatile impurities turn into vapor while the heavier biolubricant remains liquid. This thermal separation allows for the isolation of specific components based on their volatility.
The Role of Vacuum Decompression
Advanced setups, particularly rotary evaporators, often operate under vacuum. This lowers the boiling point of solvents, allowing them to evaporate rapidly at lower temperatures.
Preventing Thermal Degradation
By evaporating solvents at lower temperatures, the process protects the target product (such as isopulegyl acetate) from heat damage. This prevents thermal decomposition or unwanted isomerization, preserving the lubricant's chemical integrity.
Critical Contaminants Removed
Residual Reactants and Solvents
The process targets excess reactants like ethanol and organic solvents such as n-hexane. Removing these is vital for recovering valuable solvents for reuse and ensuring the final product is not diluted.
Reaction By-products
Distillation effectively removes side-products generated during synthesis, such as lactones. Eliminating these ensures the chemical composition matches the intended specification.
Moisture Elimination
Water is a common byproduct or contaminant in reaction mixtures. Its removal is non-negotiable, as moisture promotes oxidation and bacterial growth in lubricants.
Impact on Final Product Quality
Ensuring Flash Point Safety
Volatile contaminants like ethanol drastically lower the flash point of a lubricant, making it a fire hazard. Distillation removes these volatiles, raising the flash point to safe, operational levels.
Reducing Corrosivity
Residual water and certain chemical by-products can corrode machinery parts. By purifying the mixture, the distillation unit produces a neutral fluid that protects rather than attacks metal surfaces.
Understanding the Trade-offs
Thermal Risks
While heat is necessary for distillation, excessive temperatures can ruin the biolubricant. If the temperature exceeds the stability limit of the oil without vacuum assistance, the product may degrade or change chemically.
Energy vs. Speed
Simple distillation is generally energy-intensive and slower. Rotary evaporators offer faster solvent recovery and better efficiency but represent a higher equipment cost and complexity.
Making the Right Choice for Your Goal
To maximize the effectiveness of your refinement stage, align your equipment choice with your specific purity and stability requirements.
- If your primary focus is thermal stability: Prioritize rotary evaporation with vacuum decompression to minimize heat exposure and prevent product decomposition.
- If your primary focus is safety and longevity: Ensure your process strictly targets the removal of water and low-boiling volatiles to maximize flash point and minimize corrosivity.
Precise control during this stage acts as the final quality checkpoint, determining whether your biolubricant is merely a chemical mixture or a high-performance engineering fluid.
Summary Table:
| Feature | Simple Distillation | Rotary Evaporator (Vacuum) |
|---|---|---|
| Primary Mechanism | Atmospheric heat separation | Vacuum-assisted evaporation |
| Temperature Range | 80°C to 140°C | Lower (due to vacuum) |
| Solvent Removal | Slow/Standard | Fast/High Efficiency |
| Product Protection | Risk of thermal degradation | Minimizes heat damage |
| Key Outcome | Purity and moisture removal | High flash point & chemical integrity |
Elevate Your Biolubricant Refinement with KINTEK
Precision is the difference between a raw mixture and a high-performance fluid. At KINTEK, we empower labs and production facilities with cutting-edge thermal separation technology designed to preserve product integrity.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Rotary Evaporators, Vacuum Systems, and specialized lab high-temp furnaces, all customizable for your unique biolubricant synthesis needs. Our equipment ensures maximum solvent recovery, moisture elimination, and superior flash point safety for your final product.
Ready to optimize your distillation process? Contact KINTEK today to find the perfect solution for your lab!
Visual Guide
References
- Mohammed Alhassan, U. Shamsideen. PRODUCTION OF BIOLUBRICANT BLEND FROM JATROPHA CURCAS OIL. DOI: 10.33003/fjs-2023-0706-2168
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How does the rotary tube sintering furnace achieve high sintering efficiency? Boost Uniformity and Speed
- What types of refractory lining materials are available for rotary furnaces? Choose the Best for Your Furnace
- What makes rotary tube furnaces suitable for continuous processing? Unlock High-Volume Efficiency & Uniformity
- What are the main applications of the Rotary Tube Tilt Furnace? Ideal for Uniform Powder Processing
- What are the equipment requirements for CO2 activation? Optimize Your Tube & Rotary Furnaces
- What are the typical rotation speeds for a rotary kiln and how do they affect material retention time? Optimize Your Kiln Performance
- What is the purpose of rotary retort furnace technology? Achieve Uniform Heat Treatment for Bulk Materials
- What makes rotary furnaces environmentally friendly? Achieve Eco-Efficient Material Processing



















