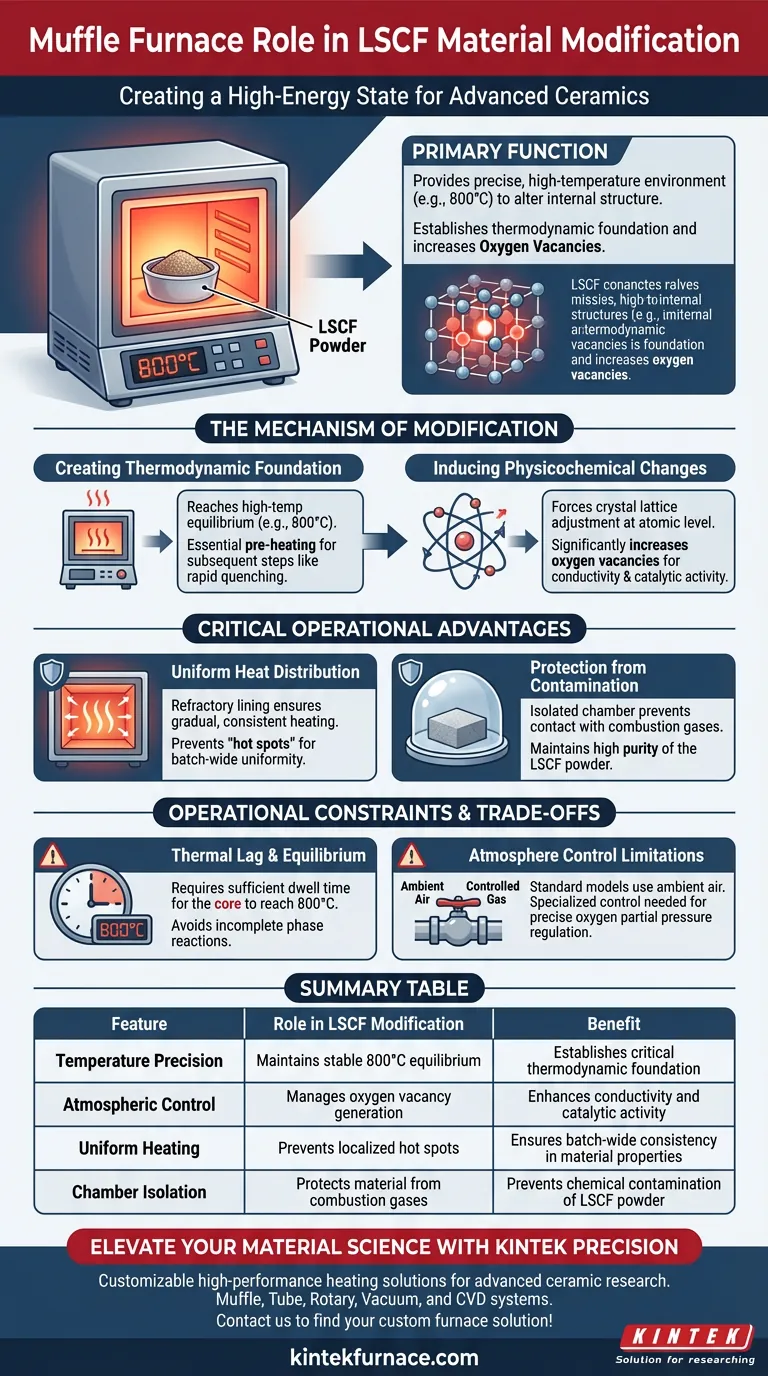
The primary function of a muffle furnace in the modification of Lanthanum Strontium Cobalt Ferrite (LSCF) is to provide a precise, high-temperature environment that alters the material's internal structure. Specifically, it heats the commercial LSCF to a target temperature, typically 800°C, to induce critical physicochemical changes before further processing.
By providing a stable thermal environment, the muffle furnace facilitates the increase of oxygen vacancies within the material. This establishes the necessary thermodynamic foundation required for subsequent treatments, such as rapid quenching.

The Mechanism of Modification
Creating a Thermodynamic Foundation
The modification of LSCF is not merely about drying or warming the material; it is about reaching a specific energy state.
The muffle furnace brings the material to a high-temperature equilibrium (e.g., 800°C). This specific thermal state acts as the thermodynamic foundation for the entire modification process. Without this precise pre-heating, subsequent steps like rapid quenching would fail to lock in the desired material properties.
Inducing Physicochemical Changes
Heat treatment in a muffle furnace triggers specific changes at the atomic level.
For LSCF, the primary goal is the increase of oxygen vacancies. By holding the material at the target temperature, the furnace forces the crystal lattice to adjust, creating these essential vacancies which significantly influence the material's conductivity and catalytic activity.
Critical Operational Advantages
Uniform Heat Distribution
Commercial modification requires consistency across the entire batch of material.
A muffle furnace is designed with a refractory lining that isolates the heating elements from the chamber. This ensures that heat radiates gradually and uniformly, preventing "hot spots" that could lead to uneven modification of the LSCF powder.
Protection from Contamination
Purity is paramount when modifying advanced ceramics like LSCF.
Because the material is isolated inside the muffle chamber, it does not come into direct contact with combustion gases or heating elements. This prevents external contaminants from altering the chemical composition of the ferrite during the delicate high-temperature phase.
Operational Constraints and Trade-offs
Thermal Lag and Equilibrium
While muffle furnaces provide stability, they do not change temperature instantly.
There is often a thermal lag between the furnace heating elements and the actual sample temperature. Operators must allow sufficient dwell time to ensure the LSCF core reaches the target 800°C, not just the furnace air. Failing to account for this results in an incomplete phase reaction.
Atmosphere Control Limitations
While muffle furnaces limit exposure to the outside environment, standard models may strictly use ambient air.
If the modification process requires a specific partial pressure of oxygen to fine-tune the vacancy concentration, the furnace must be capable of controlled atmosphere regulation. Without this, the oxidation state of the LSCF relies solely on temperature and ambient air composition, which may not be precise enough for all applications.
Making the Right Choice for Your Goal
To ensure successful modification of LSCF materials, align your furnace operation with your specific processing targets:
- If your primary focus is Defect Engineering: Ensure the furnace can maintain 800°C precisely to maximize the generation of oxygen vacancies without overheating the sample.
- If your primary focus is Process Consistency: Utilize a furnace with high-quality refractory insulation to guarantee uniform heating, ensuring the entire batch is thermodynamically prepared for quenching.
Success in LSCF modification depends not just on applying heat, but on using the muffle furnace to create a pristine, high-energy state that makes further structural manipulation possible.
Summary Table:
| Feature | Role in LSCF Modification | Benefit |
|---|---|---|
| Temperature Precision | Maintains stable 800°C equilibrium | Establishes critical thermodynamic foundation |
| Atmospheric Control | Manages oxygen vacancy generation | Enhances conductivity and catalytic activity |
| Uniform Heating | Prevents localized hot spots | Ensures batch-wide consistency in material properties |
| Chamber Isolation | Protects material from combustion gases | Prevents chemical contamination of LSCF powder |
Elevate Your Material Science with KINTEK Precision
Ready to optimize your LSCF modification process? KINTEK provides high-performance heating solutions designed for the rigorous demands of advanced ceramic research. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your specific laboratory needs.
Ensure perfect defect engineering and process consistency with our industry-leading thermal technology. Contact us today to find your custom furnace solution!
Visual Guide
References
- Ya Sun, Jian‐Qiang Wang. Controllable Technology for Thermal Expansion Coefficient of Commercial Materials for Solid Oxide Electrolytic Cells. DOI: 10.3390/ma17051216
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What features might a high-performance modern Muffle Furnace include? Discover Precision, Control, and Efficiency
- Why is a double-chamber device preferred over a standard electric furnace for sintering? Achieve Oxidation-Free Results
- How does a muffle furnace support controlled atmosphere operations? Ensure Purity and Precision in Your Lab
- How should the furnace door and samples be handled during use? Essential Safety and Maintenance Tips
- What electrical precautions should be taken when setting up a muffle furnace? Essential Safety Tips for Your Lab
- What is the role of muffle furnaces in heat treating metals? Achieve Clean, Controlled Metal Processing
- Why is controlled and consistent heating important in a muffle furnace? Ensure Reliable Results in Your Lab
- What is a hydrogen muffle furnace and how does it work? Precision Heating with Hydrogen for Oxide-Free Results



















