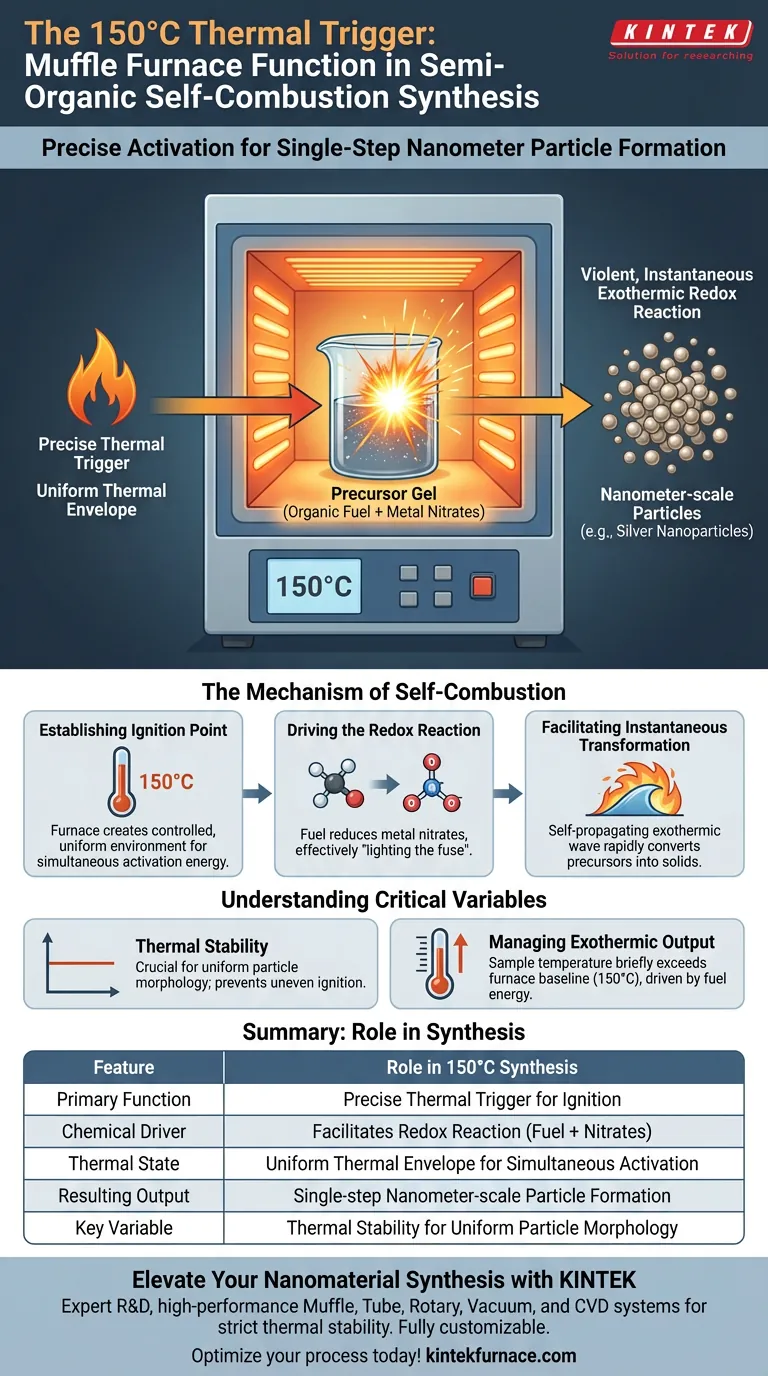
The primary function of the laboratory box resistance furnace at 150°C is to serve as a precise thermal trigger. It provides the specific ignition temperature required to initiate a redox reaction within the prepared gel. This constant heat environment allows the organic fuel and metal nitrates to undergo self-combustion, driving the synthesis process.
At this specific temperature, the furnace is not merely drying the material; it is catalyzing a violent, instantaneous exothermic reaction. This release of internal energy is the mechanic that converts the precursor gel into nanometer-scale particles in a single step.
The Mechanism of Semi-Organic Self-Combustion
To understand why the 150°C mark is critical, you must look beyond the heat source itself and look at the chemical chain reaction it initiates.
Establishing the Ignition Point
The furnace creates a controlled environment that brings the precursor gel to its critical ignition threshold.
Unlike standard drying ovens, the muffle furnace maintains a uniform thermal envelope. This uniformity ensures that the entire gel sample reaches the necessary activation energy simultaneously.
Driving the Redox Reaction
Once the gel reaches 150°C, the furnace heat facilitates a chemical interaction between two specific components: organic fuel (such as biomolecules or oxalic acid) and metal nitrates.
This interaction is a redox (reduction-oxidation) reaction. The furnace effectively "lights the fuse," causing the fuel to reduce the metal nitrates.
Facilitating Instantaneous Transformation
The reaction triggered by the furnace is exothermic, meaning it generates its own massive release of heat.
This internal heat spike creates a self-propagating combustion wave. This rapid process instantly converts the chemical precursors into solid nanometer-scale particles, such as silver nanoparticles.
Understanding the Critical Variables
While the process is described as a "one-step" synthesis, the role of the furnace introduces specific variables that must be managed to ensure success.
The Importance of Thermal Stability
The definition of a muffle furnace implies a separation between the heating element and the sample to protect it from direct flame or contamination.
At 150°C, stability is paramount. If the temperature fluctuates significantly, the ignition may be incomplete or uneven, leading to heterogeneous particle sizes rather than the desired uniform nanostructures.
Managing Exothermic Output
Because the reaction involves self-combustion, the sample itself will briefly exceed the ambient temperature of the furnace.
The furnace acts as a baseline. However, the actual synthesis temperature at the molecular level is driven by the energy density of the fuel used in the gel.
Making the Right Choice for Your Goal
When configuring your furnace for semi-organic self-combustion, consider your specific experimental objectives.
- If your primary focus is Particle Uniformity: Ensure your furnace has been calibrated to hold 150°C with minimal fluctuation, as uneven heating causes inconsistent ignition.
- If your primary focus is Process Efficiency: Rely on this method for high-throughput synthesis, as the simultaneous calcination and particle formation eliminates the need for multi-stage post-processing.
By treating the furnace as a chemical trigger rather than a simple heater, you gain control over the purity and morphology of your final nanomaterials.
Summary Table:
| Feature | Role in 150°C Synthesis |
|---|---|
| Primary Function | Precise Thermal Trigger for Ignition |
| Chemical Driver | Facilitates Redox Reaction (Fuel + Nitrates) |
| Thermal State | Uniform Thermal Envelope for Simultaneous Activation |
| Resulting Output | Single-step Nanometer-scale Particle Formation |
| Key Variable | Thermal Stability for Uniform Particle Morphology |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise temperature control is the difference between heterogeneous waste and uniform nanostructures. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the strict thermal stability required for complex redox reactions.
Whether you are conducting semi-organic self-combustion or high-temp calcination, our furnaces are fully customizable to meet your unique laboratory needs.
Ready to optimize your synthesis process? Contact us today to find your perfect furnace solution!
Visual Guide
References
- Muneeb Irshad, Martin Motola. Harnessing bio-based chelating agents for sustainable synthesis of AgNPs: Evaluating their inherent attributes and antimicrobial potency in conjunction with honey. DOI: 10.1016/j.heliyon.2024.e31424
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What design features enhance the durability and performance of a muffle furnace? Boost Lab Efficiency with Robust Design
- What happens to convective and radiative heat transfer effects at high furnace gas temperatures? Radiation Dominates for Superior Heating
- What are the different types of muffle furnaces based on heating elements? Choose the Right One for Your Lab
- How should materials be selected for use in a Muffle furnace? Optimize Your High-Temperature Processes
- What process function does a high-temperature muffle furnace perform in pre-sintering spinel ceramics?
- What should be done if the silicon carbon rod in the muffle furnace's resistance furnace ages or underperforms? Expert Tips for Optimal Performance
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What paint industry processes utilize muffle furnaces? Essential for Lab Analysis and Quality Control



















