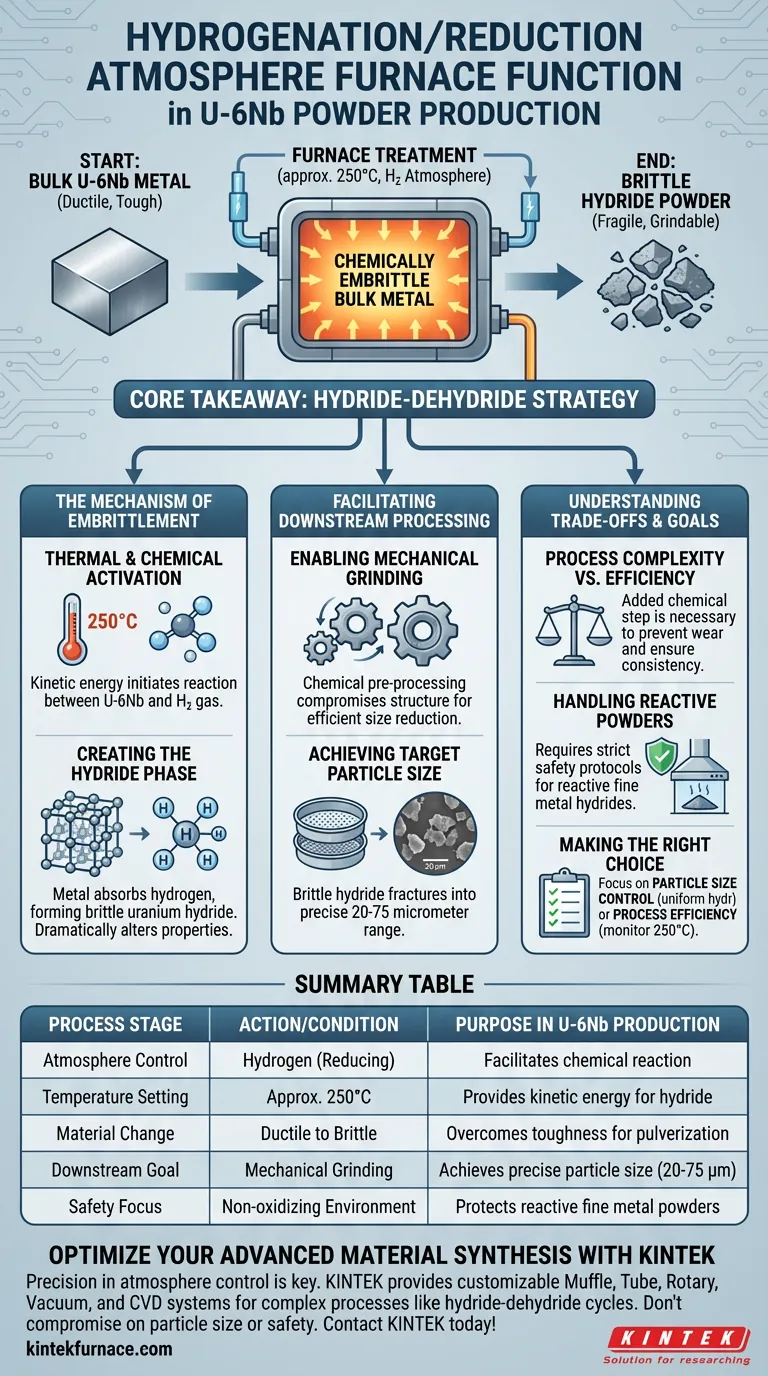
The primary function of the hydrogenation/reduction atmosphere furnace is to chemically embrittle bulk Uranium-Niobium (U-6Nb) metal to prepare it for pulverization. By exposing the metal to hydrogen gas at approximately 250°C, the furnace facilitates a reaction that converts the tough alloy into a brittle hydride powder.
Core Takeaway This process utilizes a "hydride-dehydride" strategy to overcome the natural toughness of the U-6Nb alloy. The furnace creates a controlled environment to chemically shatter the metal's structure, rendering it fragile enough to be mechanically ground into fine powder.
The Mechanism of Embrittlement
Thermal and Chemical Activation
The furnace operates at a specific set point of approximately 250°C.
At this temperature, the kinetic energy is sufficient to initiate a reaction between the bulk Uranium-Niobium metal and the hydrogen gas introduced into the chamber.
Creating the Hydride Phase
The introduction of hydrogen gas creates a reducing atmosphere essential for the transformation.
The metal absorbs the hydrogen, forming a uranium hydride. This chemical change dramatically alters the physical properties of the material, turning a ductile bulk metal into a brittle solid.
Facilitating Downstream Processing
Enabling Mechanical Grinding
The central purpose of using this furnace is to make the material grindable.
The U-6Nb alloy in its natural state is too tough for efficient mechanical size reduction. The furnace treatment acts as a chemical pre-processing step that intentionally compromises the material's structural integrity.
Achieving Target Particle Size
Once the material has been embrittled by the furnace, it can be moved to the grinding stage.
Because the hydride powder is brittle, it fractures easily and predictably. This allows operators to grind the material down to a precise target size range of 20 to 75 micrometers.
Understanding the Trade-offs
Process Complexity vs. Mechanical Efficiency
Using this furnace adds a distinct chemical processing step to the manufacturing line.
While it introduces complexity in terms of atmospheric control and temperature regulation, it is a necessary trade-off. Attempting to grind U-6Nb without this chemical embrittlement would likely result in excessive equipment wear and inconsistent particle sizes.
Handling Reactive Powders
The output of this furnace is a fine, brittle powder that is chemically distinct from the original alloy.
Handling metal hydrides requires strict adherence to safety protocols, as fine metal powders can be reactive. The "atmosphere" aspect of the furnace is critical not just for the reaction, but for maintaining a safe, non-oxidizing environment during the transition.
Making the Right Choice for Your Goal
To ensure your production line meets its specifications, consider the following based on the furnace's function:
- If your primary focus is Particle Size Control: Ensure the furnace creates a uniform hydride, as inconsistent embrittlement will make it difficult to hit the 20 to 75 micrometer target during grinding.
- If your primary focus is Process Efficiency: Monitor the 250°C temperature closely; deviations can slow the reaction rate or fail to sufficiently embrittle the bulk metal.
The hydrogenation furnace is the critical gateway that transforms a solid metal block into a workable powder form.
Summary Table:
| Process Stage | Action/Condition | Purpose in U-6Nb Production |
|---|---|---|
| Atmosphere Control | Hydrogen (Reducing) | Facilitates chemical reaction with bulk metal |
| Temperature Setting | Approx. 250°C | Provides kinetic energy for hydride formation |
| Material Change | Ductile Alloy to Brittle Hydride | Overcomes natural toughness for pulverization |
| Downstream Goal | Mechanical Grinding | Achieves precise particle size (20-75 micrometers) |
| Safety Focus | Non-oxidizing environment | Protects reactive fine metal powders |
Optimize Your Advanced Material Synthesis with KINTEK
Precision in atmosphere control is the difference between success and failure in specialized alloy production. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for complex processes like the hydride-dehydride cycle.
Whether you are processing U-6Nb or other sensitive alloys, our laboratory and industrial high-temperature furnaces are fully customizable to meet your unique temperature and atmospheric requirements. Don't compromise on particle size or safety.
Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder. DOI: 10.3390/app15126431
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the requirements for high-pressure applications in an atmosphere box furnace? Essential Guide to Safe Pressure Vessel Systems
- Why are inert atmosphere furnaces considered essential in modern industries? Unlock Purity and Precision in High-Temp Processing
- What is the role of a chemical reactor with thermogravimetric measurement in nitriding? Master AISI 1085 Kinetics
- Why is argon's inert nature important in furnace applications? Protect Materials from High-Temperature Reactions
- Purpose of High-Purity Nitrogen in Ni12P5 Synthesis: Ensuring Inert Protection and Crystal Stability
- What industries commonly use argon for heat treatment? Essential for Aerospace and High-Performance Alloys
- What environmental benefits do controlled atmosphere furnaces offer? Reduce Waste and Boost Efficiency
- How does the experimental box type atmosphere furnace contribute to energy conservation and environmental protection? Discover Sustainable Lab Solutions



















