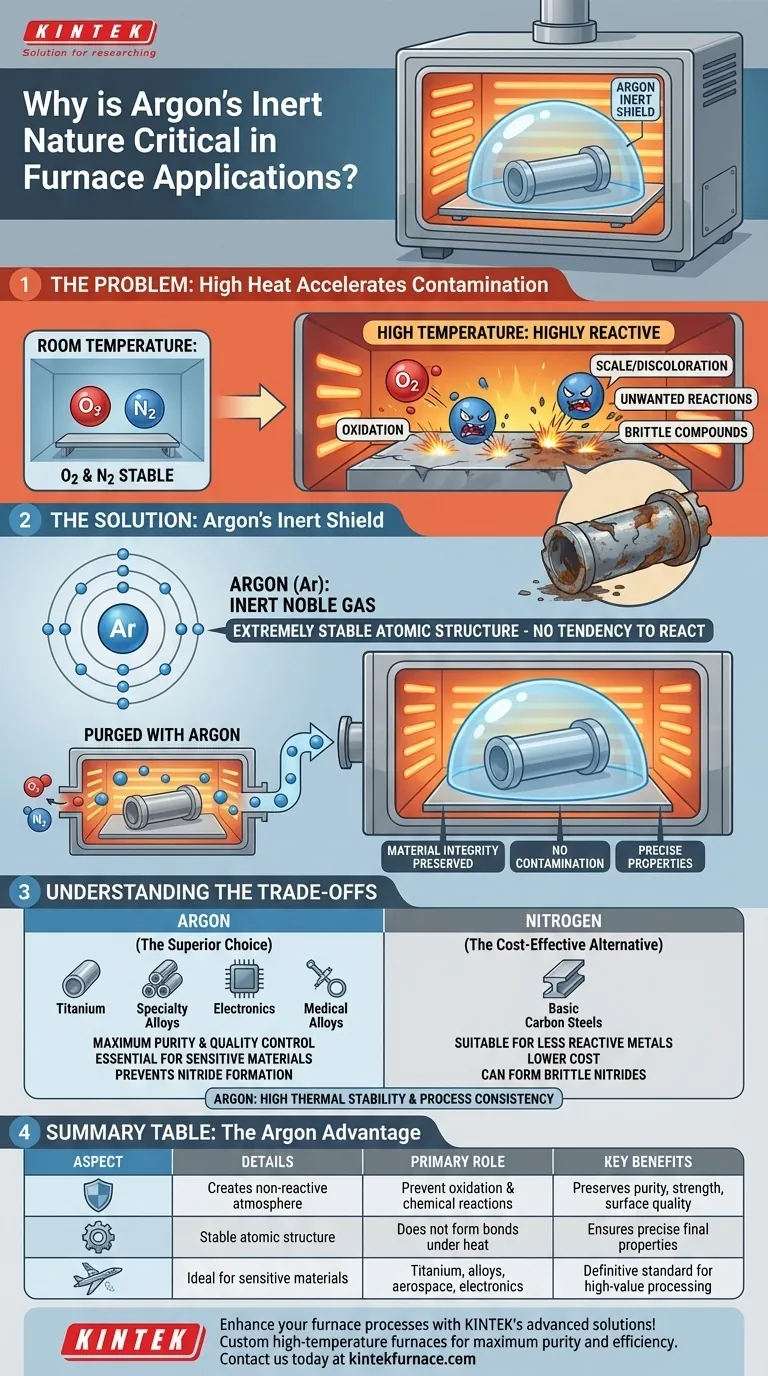
In furnace applications, argon's inert nature is critical because it creates a protective atmosphere that prevents materials from reacting with air at high temperatures. This non-reactive shield is essential for preventing damaging chemical changes like oxidation, ensuring the final product maintains its intended purity, strength, and surface quality.
At the extreme temperatures found in industrial furnaces, most materials become highly vulnerable to chemical reactions with the surrounding air. Argon acts as an invisible, non-reactive shield, displacing oxygen and other gases to guarantee the material's integrity is preserved throughout the process.

The Problem: High Heat Accelerates Contamination
At room temperature, the oxygen and nitrogen in the air are relatively stable. However, introducing the intense heat of a furnace dramatically changes their behavior.
The Aggression of Hot Air
The air we breathe is roughly 21% oxygen and 78% nitrogen. When heated, these gases become highly reactive and eager to form chemical bonds with other elements.
The Damage of Oxidation
Oxidation is the most common form of high-temperature contamination. Hot oxygen readily reacts with metal surfaces, forming a layer of oxides, commonly seen as scale or discoloration. This layer can ruin a product's surface finish, alter its dimensions, and compromise its structural integrity.
The Threat of Unwanted Reactions
Beyond just oxygen, other gases like nitrogen and water vapor can also react with materials in a furnace. These reactions can introduce impurities or form brittle compounds within the material, fundamentally altering its mechanical and chemical properties.
Argon as the Solution: The Inert Shield
Argon's value comes from what it doesn't do. As a noble gas, its atomic structure makes it extremely stable and unwilling to participate in chemical reactions.
What "Inert" Truly Means
Argon has a full outer shell of electrons, which is a highly stable atomic state. This means it has no tendency to share, gain, or lose electrons to form bonds with other elements, even under intense heat and pressure.
Purging the Environment
To be effective, argon is used to purge the furnace chamber. This process involves flooding the sealed furnace with argon gas, which is heavier than air and displaces the reactive oxygen, nitrogen, and any moisture present.
Preserving Material Integrity
Once the furnace is filled with this inert argon atmosphere, materials can be heated, melted, annealed, or welded without risk of contamination. The material is only exposed to non-reactive argon, ensuring its purity and properties remain exactly as specified.
Understanding the Trade-offs
While argon is highly effective, it's not the only option, and its use involves specific considerations.
Why Not Just Use Nitrogen?
Nitrogen is also used to create a protective atmosphere and is significantly cheaper than argon. However, it is not truly inert. At high temperatures, nitrogen can react with certain metals like titanium, aluminum, and some stainless steels to form brittle compounds called nitrides.
Argon for Sensitive Materials
For processes involving highly reactive metals or applications where even trace impurities are unacceptable—such as in electronics manufacturing or medical-grade alloys—argon is the superior and often necessary choice.
A Secondary Benefit: Thermal Stability
While its primary role is chemical protection, argon's density and low thermal conductivity also help in maintaining a more stable and uniform temperature inside the furnace. This can reduce energy consumption and improve process consistency.
Making the Right Choice for Your Process
Choosing the correct atmospheric gas is a critical decision based on material type, process requirements, and cost.
- If your primary focus is cost-effectiveness with less reactive metals: Nitrogen can be a suitable choice for processing materials like basic carbon steels.
- If your primary focus is maximum purity and quality control: Argon is the definitive standard for processing sensitive, high-value materials like titanium, specialty alloys, and components for the aerospace or semiconductor industries.
- If your primary focus is high-quality welding or metal 3D printing: Argon is essential to shield the molten metal pool from atmospheric contamination, ensuring a strong, clean, and non-porous result.
Ultimately, selecting argon is an investment in process control, guaranteeing that materials emerge from the furnace with the precise properties you designed them to have.
Summary Table:
| Aspect | Details |
|---|---|
| Primary Role | Creates a non-reactive shield to prevent oxidation and other chemical reactions at high temperatures. |
| Key Benefits | Preserves material purity, strength, and surface quality; ensures precise properties in final products. |
| Common Uses | Ideal for sensitive materials like titanium, alloys, and in aerospace, electronics, and welding applications. |
| Comparison | More effective than nitrogen for reactive metals, as it avoids nitride formation and impurities. |
Enhance your furnace processes with KINTEK's advanced solutions! Leveraging exceptional R&D and in-house manufacturing, we provide high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems, with deep customization to meet your unique needs. Ensure maximum purity and efficiency—contact us today to discuss how our tailored furnace solutions can benefit your laboratory!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance



















