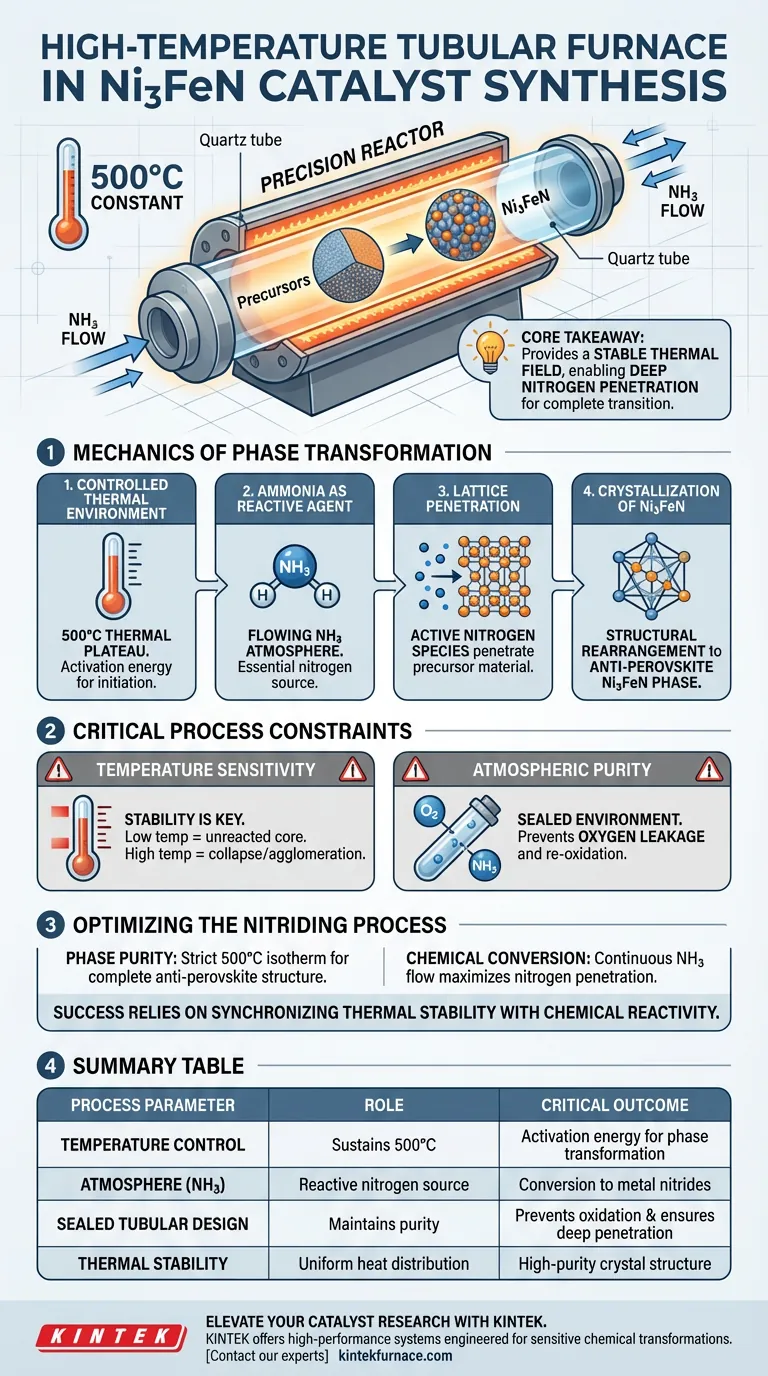
In the context of Ni3FeN catalyst synthesis, a high-temperature tubular furnace functions as a precision reactor that drives the chemical transformation of precursor materials into a specific crystal structure.
By maintaining a constant temperature of 500°C in an ammonia (NH3) atmosphere, the furnace provides the necessary thermal energy to convert metal oxides or hydroxides into the desired anti-perovskite Ni3FeN phase.
Core Takeaway The tubular furnace is not merely a heating element; it provides a stable thermal field that enables nitrogen atoms to penetrate the metal lattice. This deep penetration is the critical mechanism that facilitates the complete transition from a precursor state to a functional metal nitride catalyst.
The Mechanics of Phase Transformation
Controlled Thermal Environment
The primary role of the furnace is to establish and maintain a precise thermal plateau at 500°C.
This specific temperature is critical because it provides the activation energy required to initiate the chemical reaction without causing the material to decompose or sinter excessively.
Ammonia as the Reactive Agent
Unlike standard heating in air, this process utilizes the tubular furnace's ability to contain a flowing gas atmosphere.
Ammonia (NH3) gas is introduced into the tube, serving as the essential nitrogen source for the reaction.
Lattice Penetration
Under these high-temperature conditions, the ammonia decomposes, releasing active nitrogen species.
The stable heat ensures these nitrogen atoms possess enough energy to penetrate the metal lattice of the precursor material thoroughly.
Crystallization of Ni3FeN
As nitrogen integrates into the lattice, it forces a structural rearrangement of the atoms.
This rearrangement completes the transformation from oxides or hydroxides into the anti-perovskite Ni3FeN crystal phase, which is the definable characteristic of this specific catalyst.
Critical Process Constraints
Temperature Sensitivity
The "function" of the furnace relies heavily on stability; deviations from the 500°C target can ruin the catalyst.
If the temperature is too low, the nitrogen penetration will be superficial, leaving an unreacted oxide core. If too high, the crystal structure may collapse or agglomerate, reducing surface area.
Atmospheric Purity
The tubular design must effectively seal the environment to maintain a pure ammonia atmosphere.
Any leakage of oxygen into the tube during this phase would counteract the nitriding process, leading to re-oxidation rather than the formation of the desired nitride.
Optimizing the Nitriding Process
To ensure the high-temperature tubular furnace delivers the correct catalytic properties, focus on the specific parameters of your synthesis goal:
- If your primary focus is Phase Purity: Ensure the furnace maintains a strict 500°C isotherm to guarantee the complete formation of the anti-perovskite structure without secondary phases.
- If your primary focus is Chemical Conversion: Verify the continuous flow and concentration of NH3 gas to maximize nitrogen penetration into the precursor lattice.
The success of Ni3FeN synthesis relies entirely on the furnace's ability to synchronize thermal stability with chemical reactivity.
Summary Table:
| Process Parameter | Role in Ni3FeN Synthesis | Critical Outcome |
|---|---|---|
| Temperature Control | Sustains 500°C isothermal plateau | Activation energy for phase transformation |
| Atmosphere (NH3) | Reactive nitrogen source | Conversion of precursors to metal nitrides |
| Sealed Tubular Design | Maintains atmospheric purity | Prevents oxidation and ensures deep lattice penetration |
| Thermal Stability | Uniform heat distribution | High-purity anti-perovskite crystal structure |
Elevate Your Catalyst Research with KINTEK
Precision is non-negotiable when synthesizing high-performance Ni3FeN catalysts. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, and Vacuum systems specifically engineered for sensitive chemical transformations. Our systems provide the stable thermal fields and atmospheric integrity essential for nitrogen lattice penetration and crystal purity. Whether you need standard or fully customizable lab high-temp furnaces, KINTEK ensures your unique synthesis requirements are met with industry-leading reliability. Contact our experts today to optimize your nitriding process!
Visual Guide
References
- Yunxiang Lin, Li Song. Optimizing surface active sites via burying single atom into subsurface lattice for boosted methanol electrooxidation. DOI: 10.1038/s41467-024-55615-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the specific role of a tube furnace in the pre-treatment of activated carbon catalysts? Precision Modification
- How do three-zone tube furnaces support scalability? Bridge Lab to Industrial Production Seamlessly
- How does a multi-tube pyrolysis furnace achieve precise temperature control? Advanced Hardware & PID Logic Explained
- What factors determine the selection of a three-zone split tube furnace? Key Specs for Precision Thermal Processing
- What are the specifications for three-zone and three-phase horizontal tube furnace models? Find the Perfect Fit for Your Lab
- What are the benefits of using a vertical tube furnace? Unlock Superior Homogeneity for Cobalt/Carbon Supports
- What is the role of a tube furnace system in the growth of bilayer MoS2? Master CVD Synthesis with Precision Control
- What role does a tube furnace play in gas-phase nitridation? Transform TiO2 with Precise Nitrogen Doping



















