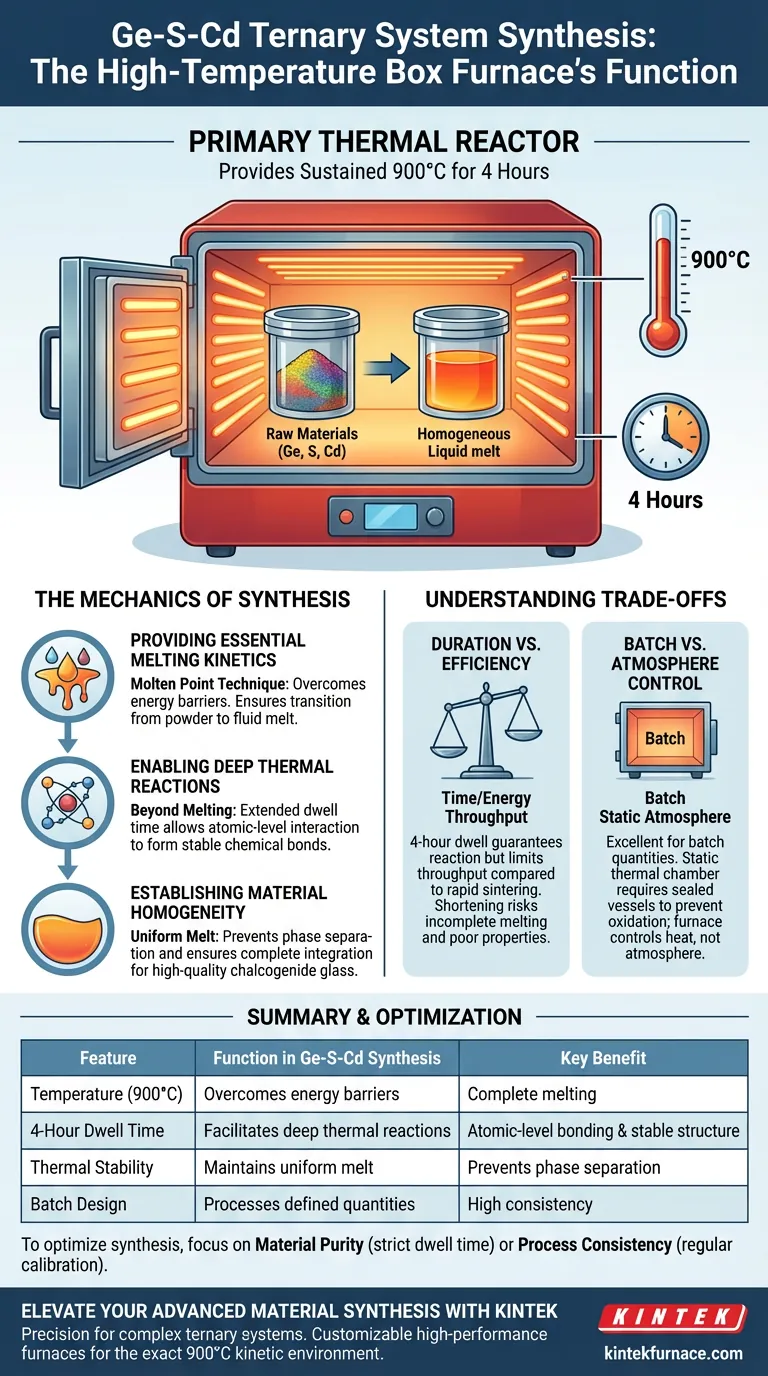
The high-temperature box furnace serves as the primary thermal reactor for synthesizing the Ge-S-Cd ternary system. Its specific function is to provide a sustained environment of 900 degrees Celsius for four hours, which supplies the necessary kinetic energy for the raw materials to melt completely and react chemically.
The furnace does not simply heat the material; it creates a specific "melting kinetic environment" that allows elements to transition from a solid mixture into a homogeneous liquid, enabling the stable chemical bonding essential for high-quality chalcogenide glass ingots.
The Mechanics of Ge-S-Cd Synthesis
Providing Essential Melting Kinetics
The synthesis of the Ge-S-Cd system relies on the "molten point technique." The high-temperature box furnace is responsible for overcoming the energy barriers of the solid raw materials.
By maintaining a steady temperature of 900°C, the furnace ensures that Germanium, Sulfur, and Cadmium components reach their respective melting points. This thermal load is critical to transition the physical state of the mixture from a powder or aggregate into a fluid melt.
Enabling Deep Thermal Reactions
Beyond simple melting, the furnace facilitates complex chemical interactions. The extended duration of the heating cycle is just as critical as the temperature itself.
Holding the system at temperature for four hours allows for "deep thermal reactions." This dwell time ensures that the elements do not just mix physically but interact at the atomic level to form stable chemical bonds.
Establishing Material Homogeneity
The ultimate goal of this thermal process is to produce a high-quality ternary chalcogenide glass ingot. The box furnace ensures the melt becomes uniform throughout.
Without this sustained high-temperature environment, the resulting material would likely suffer from phase separation or incomplete integration of the Cadmium into the Ge-S matrix.
Understanding the Trade-offs
Process Duration vs. Efficiency
The four-hour dwell time at 900°C is a significant energy and time investment. While it guarantees complete reaction, it limits the throughput of the manufacturing process compared to rapid-sintering techniques.
Shortening this period to save energy risks incomplete melting. This often results in weak structural integrity or poor optical properties in the final glass ingot.
Batch Processing vs. Atmosphere Control
Box furnaces are typically designed for batch processing, making them excellent for preparing defined quantities of ingots.
However, unlike tube furnaces which excel at precise, flowing atmosphere control (such as constant inert gas streams), standard box furnaces function as static thermal chambers. For Ge-S-Cd synthesis, this means the containment of the sample (often in sealed vessels) is critical to prevent oxidation, as the furnace itself primarily controls heat, not the chemical atmosphere.
Optimizing Your Synthesis Strategy
To ensure the successful preparation of Ge-S-Cd ingots, apply the following principles based on your specific objectives:
- If your primary focus is Material Purity: Adhere strictly to the four-hour dwell time to ensure all volatile components react fully and stable bonds are formed.
- If your primary focus is Process Consistency: Regularly calibrate the furnace to ensure the internal chamber actually reaches and maintains 900°C without significant fluctuations.
Successful synthesis relies not just on reaching high temperatures, but on maintaining the precise kinetic environment required for stable chemical bonding.
Summary Table:
| Feature | Function in Ge-S-Cd Synthesis | Key Benefit |
|---|---|---|
| Temperature (900°C) | Overcomes energy barriers for Ge, S, and Cd | Complete melting of raw materials |
| 4-Hour Dwell Time | Facilitates deep thermal reactions | Atomic-level bonding & stable structure |
| Thermal Stability | Maintains uniform melt environment | Prevents phase separation in glass ingots |
| Batch Design | Processes defined quantities | High consistency for material research |
Elevate Your Advanced Material Synthesis with KINTEK
Precision is non-negotiable when synthesizing complex ternary systems like Ge-S-Cd. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to provide the exact 900°C kinetic environment your research demands. Whether you need static thermal chambers or precise atmosphere control, our lab high-temp furnaces ensure material homogeneity and superior structural integrity.
Ready to optimize your synthesis workflow? Contact us today to find your custom furnace solution!
Visual Guide
References
- Zainab Abd Al-hadi, Kareem A. Jasim. The Effect of Partial Substitution of Ge-S-Cd Alloys on the Density of Energy States. DOI: 10.30526/37.1.3314
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What types of heating elements are commonly used in box furnaces? Optimize Your High-Temp Processes
- Why is a muffle furnace used to determine the ash content of biochar? Master Your Material Purity Analysis
- What are the primary applications of muffle furnaces in materials research? Unlock Precision in Synthesis and Analysis
- How is a muffle furnace used in environmental analysis? Achieve Accurate Sample Preparation for Pollutants
- Why are box furnaces important in scientific research? Unlock Precision and Control for Breakthroughs
- How is temperature controlled in a box type electric furnace? Master Precise Heat Regulation for Your Lab
- Why is precise temperature control critical when sintering 13-93 bioactive glass? Expert Thermal Management Guide
- How do muffle furnaces contribute to drug testing in pharmaceuticals? Ensure Purity and Compliance with Precision



















