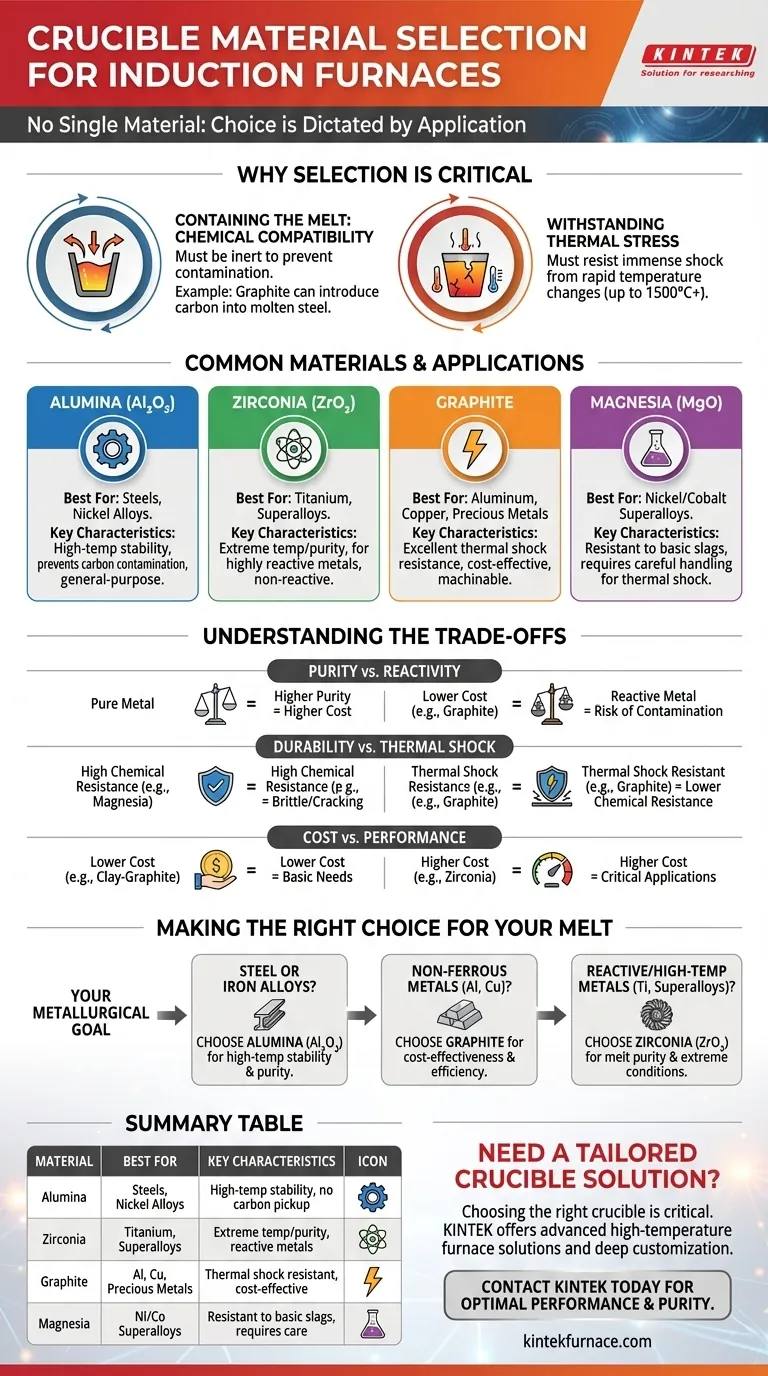
There is no single material for an induction furnace crucible; the choice is dictated entirely by the application. The most common materials include refractory ceramics like alumina and zirconia, as well as graphite, each selected based on the specific metal being melted, the required temperature, and the potential for chemical reaction.
The crucible is not a passive container. It is an active component in the melting process whose material must be chosen to ensure chemical compatibility with the molten metal and survive extreme thermal shock to prevent melt contamination and catastrophic failure.

Why Crucible Selection is Critical
The crucible serves two primary functions: physically containing the molten metal and withstanding the intense, rapid heating cycles inherent to induction melting. A failure in either of these roles compromises the entire process.
Containing the Melt: Chemical Compatibility
The crucible material must be chemically inert with respect to the alloy being melted. A reactive crucible can leach elements into the melt, introducing impurities that alter the final metal's properties.
For example, while graphite is an effective crucible material, it can react with molten iron to form iron carbide, increasing the carbon content of the steel. This makes it unsuitable for melting low-carbon steels.
Withstanding Thermal Stress
Induction furnaces heat metal with extreme speed. This subjects the crucible to immense thermal stress and shock as it goes from room temperature to over 1500°C (2732°F) and back down.
The material must have a low coefficient of thermal expansion and high fracture toughness to resist cracking or shattering during these rapid temperature changes.
Common Crucible Materials and Their Applications
Choosing the right material involves matching its properties to the demands of the metal and the process.
Alumina (Al₂O₃)
Alumina is a highly stable and widely used refractory ceramic. Its high-temperature stability and chemical inertness make it an excellent general-purpose choice, especially for ferrous metals.
It is the standard recommendation for melting steels and many nickel-based alloys where carbon contamination from a graphite crucible would be detrimental.
Zirconia (ZrO₂)
Zirconia offers superior performance at even higher temperatures than alumina. It is also exceptionally stable and non-reactive.
This makes it the material of choice for melting highly reactive metals like titanium or superalloys that require extreme temperatures and purity.
Graphite
Graphite has excellent thermal conductivity and is resistant to thermal shock. It is also easily machined and relatively cost-effective.
It is commonly used for melting many non-ferrous metals like aluminum, copper, and precious metals. However, its use with ferrous metals is limited due to the risk of carbon pickup.
Magnesia (MgO)
Magnesia is used for specific applications, particularly in melting nickel-based or cobalt-based superalloys. It offers good resistance to basic slags.
Its primary drawback is a higher susceptibility to thermal shock compared to other materials, requiring more careful heating and cooling protocols.
Understanding the Trade-offs
The ideal crucible does not exist; every material choice is a compromise between performance, cost, and operational constraints.
Purity vs. Reactivity
The primary trade-off is ensuring the crucible doesn't contaminate the melt. A graphite crucible might be thermally superior and cheaper, but if it introduces unwanted carbon into a specialty steel, it has failed its most critical task. Purity requirements often dictate the use of more expensive ceramic crucibles like alumina or zirconia.
Durability vs. Thermal Shock Resistance
Some of the most chemically resistant and high-temperature materials can be brittle. Magnesia, for example, has excellent chemical properties for certain alloys but is more prone to cracking from thermal shock than graphite. This requires operators to be more careful and can lead to a shorter service life if not handled correctly.
Cost vs. Performance
There is a direct correlation between cost and performance. A clay-graphite crucible may be sufficient for a small-scale aluminum foundry, but a zirconia crucible is non-negotiable for producing high-purity titanium aerospace components, despite being significantly more expensive.
Making the Right Choice for Your Melt
Your selection should be guided by a clear understanding of your metallurgical goal.
- If your primary focus is melting steel or iron alloys: Choose alumina for its high-temperature stability and non-reactive nature, which prevents carbon contamination.
- If your primary focus is non-ferrous metals like aluminum or copper: Graphite or a clay-graphite composite is often the most cost-effective and thermally efficient choice.
- If your primary focus is reactive or very high-temperature metals (e.g., titanium, superalloys): Zirconia is the necessary selection to ensure melt purity and withstand extreme process conditions.
Ultimately, selecting the correct crucible material is a foundational decision that directly impacts the quality, purity, and success of your melting operation.
Summary Table:
| Material | Best For | Key Characteristics |
|---|---|---|
| Alumina (Al₂O₃) | Steels, Nickel Alloys | High-temperature stability, prevents carbon contamination |
| Zirconia (ZrO₂) | Titanium, Superalloys | Extreme temperature/purity, for reactive metals |
| Graphite | Aluminum, Copper, Precious Metals | Excellent thermal shock resistance, cost-effective |
| Magnesia (MgO) | Nickel/Cobalt Superalloys | Resistant to basic slags, requires careful handling |
Need a Crucible Solution Tailored to Your Specific Melting Process?
Choosing the right crucible material is critical for achieving the desired metal purity and preventing costly contamination or crucible failure. At KINTEK, we understand that every melting operation is unique.
Leveraging our exceptional R&D and in-house manufacturing capabilities, we provide diverse laboratories and foundries with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet your unique experimental and production requirements.
Let our experts help you select or custom-engineer the perfect crucible and furnace system for your application.
Contact KINTEL today to discuss your project and ensure optimal performance and purity in your melting operations.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the primary function of a muffle furnace for BaTiO3? Master High-Temp Calcination for Ceramic Synthesis
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating
- Why is a high-performance muffle furnace required for the calcination of nanopowders? Achieve Pure Nanocrystals
- What is the role of a muffle furnace in the study of biochar regeneration and reuse? Unlock Sustainable Water Treatment
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation



















