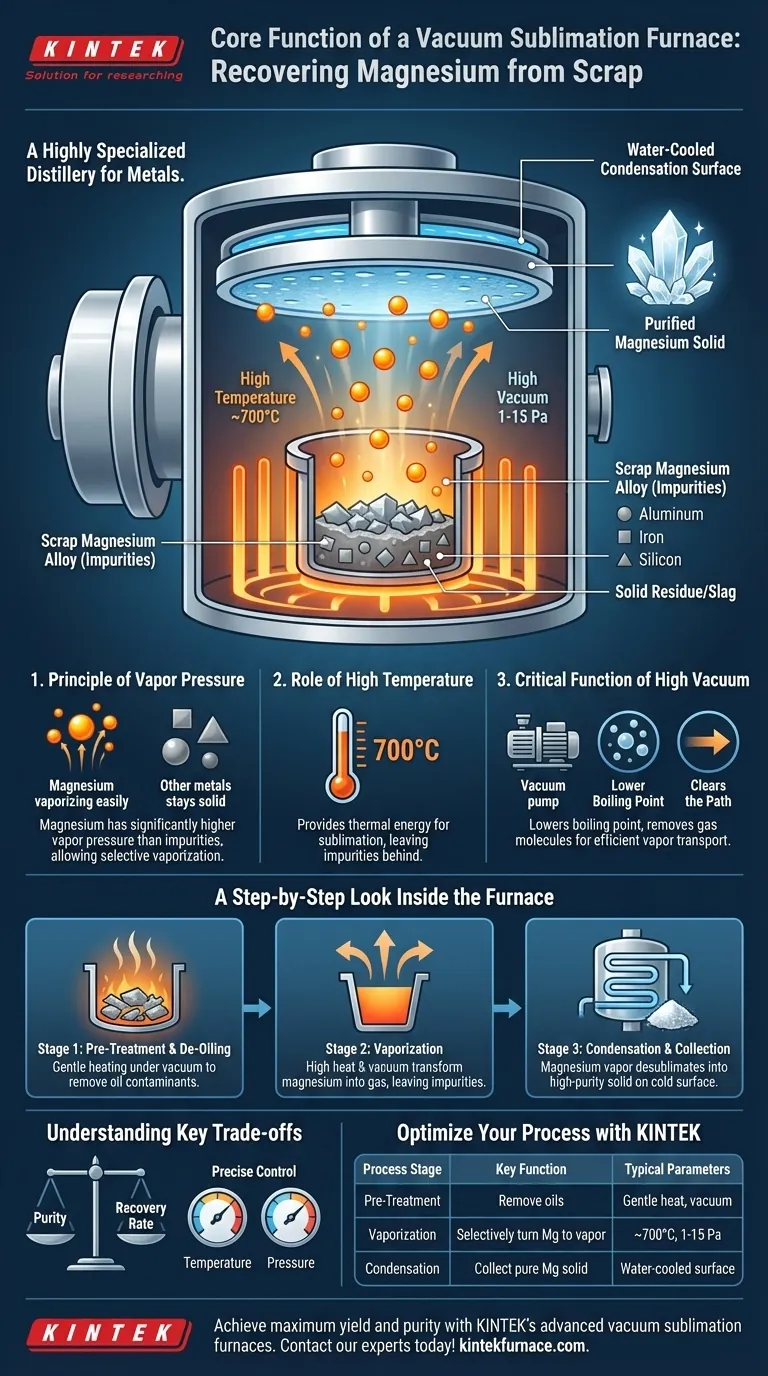
The core function of a vacuum sublimation furnace is to create a highly controlled environment of high temperature and high vacuum. This environment exploits the differences in vapor pressure between metals, allowing valuable magnesium to selectively vaporize away from scrap alloy impurities, which are left behind as solid residue.
A vacuum furnace acts as a highly specialized distillery for metals. It uses a powerful vacuum to dramatically lower magnesium's boiling point and controlled heat to turn it into a vapor, which then condenses elsewhere as a purified solid, achieving efficient separation from other materials.

How Vacuum Sublimation Achieves Separation
The entire process hinges on a fundamental principle of physics: different materials turn into vapor at different temperatures. A vacuum furnace is engineered to manipulate this principle with precision to isolate one specific metal.
The Principle of Vapor Pressure
Every element has a unique tendency to vaporize at a given temperature, a property known as vapor pressure. Magnesium has a significantly higher vapor pressure than common alloy impurities like aluminum, iron, and silicon.
This means that under the right conditions, magnesium will readily turn into a gas while the other elements remain in a liquid or solid state, forming the basis for their separation.
The Role of High Temperature
The furnace’s heating system provides the critical thermal energy needed for sublimation or vaporization to occur.
The scrap alloy, held in a crucible, is heated to a precise temperature, often around 700°C (1373 K). This is hot enough to give the magnesium molecules the energy they need to escape as a vapor but cool enough to leave most impurities behind.
The Critical Function of High Vacuum
Creating a high-vacuum environment, typically at a pressure of 1-15 Pa, is the most crucial step and serves two distinct purposes.
First, it dramatically lowers the boiling point of magnesium. Just as water boils at a lower temperature at high altitudes, magnesium vaporizes much more easily in a vacuum, making the process faster and more energy-efficient.
Second, the vacuum removes air and other gas molecules, which clears the path for the magnesium vapor. This increases the "mean free path," allowing the vapor to travel directly to the collection surface without collisions, maximizing the recovery rate and final purity.
A Step-by-Step Look Inside the Furnace
The recovery of magnesium is a multi-stage process that occurs entirely within the controlled environment of the furnace.
Pre-Treatment and De-Oiling
Scrap magnesium is often contaminated with oils from machining. Before sublimation, the furnace can be used to gently heat the material under vacuum to vaporize and extract these oils, preventing them from breaking down into carbon and contaminating the final product.
Vaporization in the Crucible
Once the scrap is clean, the furnace temperature is increased to the target for sublimation. The combination of heat and vacuum causes the pure magnesium to rapidly transform into a gas, leaving the less volatile impurities behind in the crucible as a slag.
Condensation and Collection
The hot magnesium vapor rises and makes contact with a much cooler surface inside the furnace. This is typically a specially designed, water-cooled condensation disc or the furnace lid itself.
Upon contact with this cold surface, the magnesium vapor instantly desublimates—turning directly from a gas back into a high-purity solid—forming crystalline deposits of refined magnesium.
Understanding the Key Trade-offs
Achieving optimal results requires a deep understanding of the process parameters and their interplay, as small deviations can significantly impact the outcome.
Purity vs. Recovery Rate
There is often a trade-off between the purity of the recovered magnesium and the total amount recovered (the yield). Operating at higher temperatures can increase the recovery rate but also risks vaporizing some impurities with closer boiling points, slightly reducing purity.
The Need for Precise Control
The process is highly sensitive to both temperature and pressure. If the vacuum is not strong enough, the efficiency of vapor transport drops. If the temperature is not controlled precisely, you can either fail to vaporize enough magnesium or accidentally vaporize unwanted elements.
The Impact of Contaminants
The importance of the de-oiling pre-treatment cannot be overstated. Any organic compounds left on the scrap will contaminate the final product, defeating the purpose of the purification process and requiring additional, costly refining steps.
Applying This to Your Goal
The operational parameters of the furnace should be tuned based on the specific objective of the recovery process.
- If your primary focus is maximizing purity: Maintain the lowest possible temperature that still allows for efficient magnesium vaporization and ensure the highest vacuum to prevent any co-vaporization of impurities.
- If your primary focus is maximizing recovery rate: Operate at the higher end of the acceptable temperature range and maintain the highest vacuum level possible to ensure nearly all magnesium vapor reaches the condenser.
- If your primary focus is overall process efficiency: Implement a thorough de-oiling pre-treatment step to guarantee a high-quality product in a single run, avoiding rework and contamination.
By precisely controlling these physical conditions, a vacuum sublimation furnace transforms low-value scrap into a high-purity, high-value industrial resource.
Summary Table:
| Process Stage | Key Function | Typical Parameters |
|---|---|---|
| Pre-Treatment (De-oiling) | Removes oils and contaminants from scrap | Gentle heat under vacuum |
| Vaporization | Selectively turns magnesium into vapor | ~700°C, 1-15 Pa vacuum |
| Condensation | Collects pure magnesium as a solid | Contact with water-cooled surface |
| Result | High-purity magnesium separated from impurities | Purity vs. Recovery Rate trade-off |
Optimize Your Magnesium Recovery Process with KINTEK
Recovering high-purity magnesium from scrap alloys requires precise control over temperature and vacuum. KINTEK's advanced vacuum sublimation furnaces are engineered to deliver this critical performance, ensuring maximum yield and purity for your specific application—whether your priority is ultimate product quality, highest recovery rate, or overall process efficiency.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD furnace systems, all customizable for your unique needs.
Contact our experts today to discuss how a KINTEK vacuum furnace can transform your scrap into a valuable resource.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How is the structure of a rotary tube furnace characterized? Discover Its Key Components and Benefits
- Why is efficient heat transfer important in rotary tube furnaces? Boost Uniformity and Throughput
- What are some applications of rotary tube furnaces? Ideal for Continuous High-Temperature Material Processing
- What are the benefits of continuous sample movement in rotary tube furnaces? Boost Uniformity and Efficiency
- What are the key features of a rotary furnace? Achieve Superior Uniformity and Control



















