In technical terms, a protective atmosphere is a controlled gaseous environment used to replace the ambient air surrounding a product. This is done to prevent or slow down the unwanted chemical and biological reactions that cause degradation. For example, in food packaging, the air inside a package is replaced with a specific gas mixture to stop the food from spoiling, losing its color, or changing in texture.
The core principle of a protective atmosphere is the removal of reactive elements—primarily oxygen and moisture—from a product's environment. By replacing them with a stable, often inert gas, you can dramatically slow down processes like oxidation, spoilage, and corrosion.

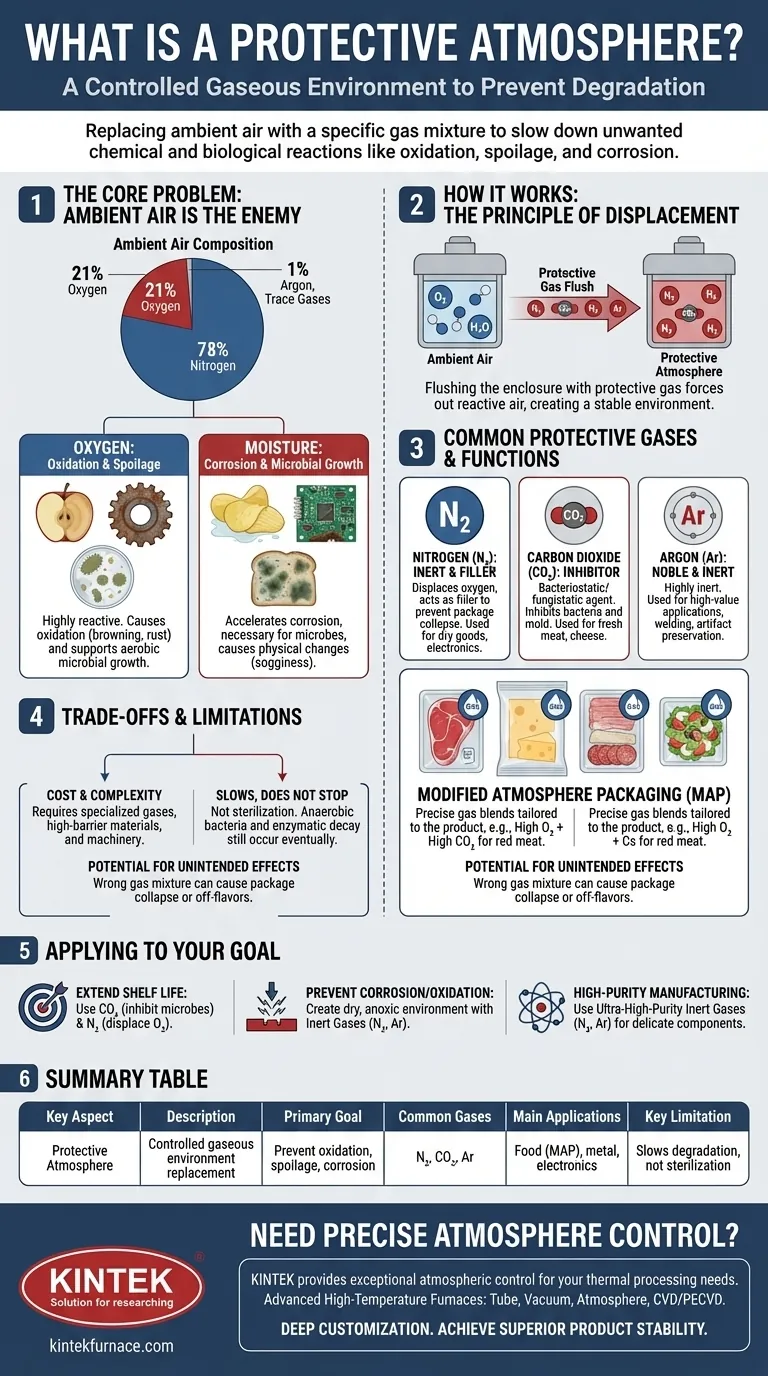
The Core Problem: Why Air Is Often the Enemy
To understand why a protective atmosphere is necessary, you must first understand the components of the air we breathe and how they interact with materials. Air is roughly 78% nitrogen, 21% oxygen, 1% argon, and trace amounts of other gases, including water vapor.
The Role of Oxygen: Oxidation and Spoilage
Oxygen is a highly reactive element. While essential for life, it is a primary driver of degradation for many products.
This process, called oxidation, is responsible for the browning of a cut apple, the rusting of iron, and the rancid taste that develops in fatty foods. Oxygen also supports the growth of aerobic microorganisms, like many types of bacteria and mold, which cause food to spoil.
The Impact of Moisture: Corrosion and Microbial Growth
Water vapor present in the air is another major factor in degradation. It can accelerate the corrosion of metals and is a necessary component for most microbial growth.
Even in the absence of oxygen, moisture can cause physical changes, such as making a crisp snack go soft. Controlling moisture is just as critical as controlling oxygen.
How a Protective Atmosphere Works
A protective atmosphere solves these problems by systematically replacing the problematic ambient air with a carefully selected gas or gas mixture tailored to the specific product.
The Principle of Displacement
The fundamental technique is to flush the package or enclosure with the desired protective gas, forcing the ambient air out. This process creates an environment dominated by the new, non-reactive or beneficial gas.
Common Protective Gases and Their Functions
Different gases are chosen for their unique properties. The most common are:
- Nitrogen (N₂): As an inert gas, nitrogen does not easily react with other substances. Its main function is to displace oxygen and act as a filler gas to prevent package collapse. It's used extensively for dry goods like potato chips and in electronics manufacturing.
- Carbon Dioxide (CO₂): Beyond displacing oxygen, carbon dioxide is a powerful bacteriostatic and fungistatic agent, meaning it actively inhibits the growth of bacteria and molds. This makes it essential for preserving products like fresh meat and cheeses.
- Argon (Ar): A noble gas, argon is even more inert than nitrogen. It is used in high-value applications where even the slightest reaction must be prevented, such as in welding high-alloy steels, preserving historical artifacts, and in certain wine preservation systems.
Modified Atmosphere Packaging (MAP)
In the food industry, this technology is most famously known as Modified Atmosphere Packaging (MAP). It often uses a precise blend of these gases. For instance, a package of red meat might contain high levels of oxygen to maintain its bright red color, combined with high levels of carbon dioxide to inhibit microbial growth.
Understanding the Trade-offs and Limitations
While highly effective, implementing a protective atmosphere is not without its challenges and considerations. It is a preservation method, not a sterilization method.
Cost and Complexity
Using specialized gases, packaging materials with high barrier properties, and the machinery required to perform the gas flushing all add cost and complexity to the production process.
It Slows, It Does Not Stop
A protective atmosphere significantly extends shelf life but does not stop the aging process entirely. Anaerobic bacteria can still grow, and natural enzymatic decay will eventually occur. The initial quality of the product remains the most important factor.
Potential for Unintended Effects
Using the wrong gas mixture can have negative consequences. For example, high concentrations of carbon dioxide can be absorbed by some foods, causing the package to collapse or leading to a slightly acidic taste.
Applying This to Your Goal
The right protective atmosphere strategy depends entirely on what you are trying to protect and why.
- If your primary focus is extending food shelf life: You will likely use a blend of Carbon Dioxide to inhibit microbial growth and Nitrogen to displace oxygen and provide bulk.
- If your primary focus is preventing metal corrosion or oxidation: Your goal is to create a dry, anoxic environment using an inert gas like Nitrogen or Argon to eliminate both oxygen and moisture.
- If your primary focus is high-purity manufacturing (e.g., electronics): You will use ultra-high-purity inert gases like Nitrogen or Argon to prevent even microscopic oxidation that could compromise delicate components.
By understanding and controlling the atmosphere, you gain direct control over the stability and longevity of your product.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Goal | Replace ambient air to prevent unwanted chemical/biological reactions (oxidation, spoilage, corrosion). |
| Core Principle | Displacement of reactive elements, primarily oxygen and moisture, with stable/inert gases. |
| Common Gases | Nitrogen (N₂), Carbon Dioxide (CO₂), Argon (Ar). |
| Main Applications | Food packaging (MAP), metal processing, electronics manufacturing, artifact preservation. |
| Key Limitation | Slows degradation but does not stop it entirely; not a sterilization method. |
Need to Create a Precise Protective Atmosphere for Your Process?
Whether you are extending food shelf life, preventing metal corrosion, or ensuring high-purity manufacturing, the right thermal processing environment is critical. KINTEK's advanced high-temperature furnaces—including Tube, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—are engineered to provide exceptional atmospheric control.
Leveraging our strong in-house R&D and manufacturing capabilities, we offer deep customization to precisely meet your unique experimental and production requirements.
Let us help you achieve superior product stability and longevity. Contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance



















