At its core, a controlled atmosphere for heat treatment is a specifically engineered gaseous environment inside a furnace that replaces ambient air. Its purpose is to actively manage the chemical reactions that occur on a metal's surface at high temperatures. This precise control prevents undesirable effects like oxidation and decarburization, ensuring the heat treatment process achieves its intended metallurgical goals without compromising the component's surface integrity.
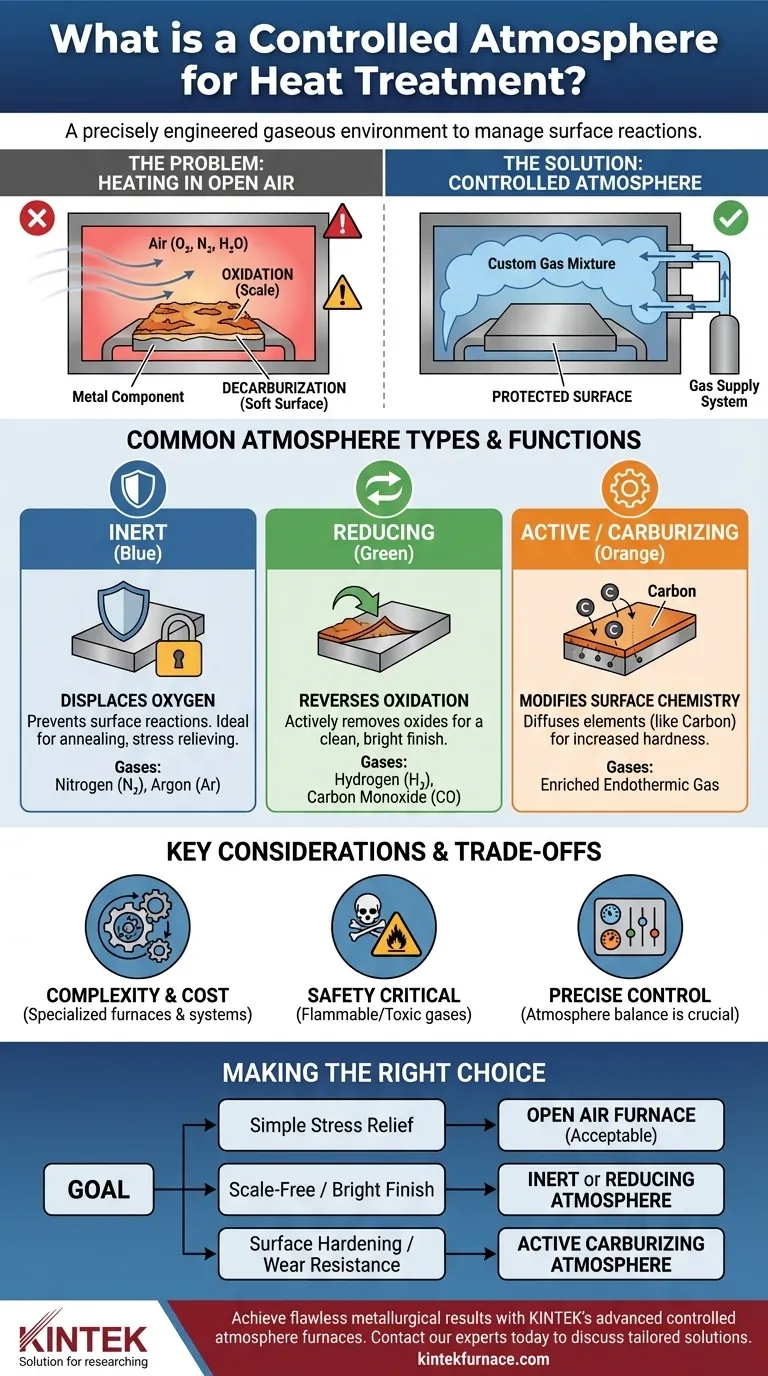
The fundamental challenge of heat treatment is that heating metal in open air causes destructive surface reactions. A controlled atmosphere solves this by replacing reactive air with a custom gas mixture, protecting the part and enabling precise control over the final surface properties.

The Fundamental Problem: Why Air is the Enemy
When metals are heated to high temperatures, they become highly reactive with the gases in the atmosphere. Standard air, composed primarily of nitrogen, oxygen, and water vapor, is particularly aggressive.
The Challenge of Oxidation
At elevated temperatures, oxygen in the air rapidly reacts with the surface of most metals, especially steel. This reaction forms a layer of metallic oxide, commonly known as scale.
This scale is problematic because it alters the component's dimensions, creates a poor surface finish, and must often be removed through costly secondary operations like sandblasting or acid pickling.
The Risk of Decarburization
For carbon and alloy steels, another destructive reaction occurs. The carbon atoms near the surface of the steel can react with oxygen and water vapor in the air, effectively removing carbon from the surface layer.
This loss of carbon, known as decarburization, creates a soft, weak surface on a part that was intended to be hard. This severely compromises the component's wear resistance and fatigue life.
How a Controlled Atmosphere Solves the Problem
A controlled atmosphere system works by purging the furnace of air and replacing it with a gas or mixture of gases that is either non-reactive or beneficially reactive with the metal.
Creating a Protective Shield
The most basic function of a controlled atmosphere is to displace the oxygen. By filling the furnace with a gas that does not react with the metal, the part is shielded from oxidation and decarburization throughout the heating and cooling cycle.
Common Atmosphere Types and Their Functions
The specific gas composition is chosen based on the metal being treated and the desired outcome.
-
Inert Atmospheres: Gases like Nitrogen and Argon are chemically inert. Their sole purpose is to displace oxygen and prevent any surface reactions. This is ideal for processes like annealing or stress relieving where the goal is to alter the metal's internal structure without changing its surface.
-
Reducing Atmospheres: These atmospheres, often containing Hydrogen and Carbon Monoxide (e.g., Endothermic Gas), are chemically active. They not only displace oxygen but also actively "reduce" or reverse any oxides that may have been present on the part's surface, resulting in a clean, bright finish.
-
Active or Carburizing Atmospheres: Some processes use the atmosphere to intentionally change the surface chemistry. In carburizing, a gas mixture rich in carbon potential (like Endothermic gas enriched with natural gas) is used to diffuse carbon atoms into the surface of a steel part, creating a hard, wear-resistant "case."
Understanding the Trade-offs
While highly effective, controlled atmosphere heat treatment is not a universal solution. It introduces complexities that must be carefully managed.
Increased Cost and Complexity
Furnaces designed for controlled atmospheres are more complex and expensive than simple air furnaces. They require sealed chambers, sophisticated gas generation and mixing systems, and precise monitoring equipment to maintain the correct atmosphere composition.
Critical Safety Considerations
Many atmospheres involve gases that are either flammable (Hydrogen), toxic (Carbon Monoxide), or both. Operating these systems requires strict safety protocols, ventilation, and monitoring to protect personnel and facilities.
Process Control is Non-Negotiable
The "control" aspect is paramount. An improperly balanced atmosphere can be worse than using no atmosphere at all. For example, an atmosphere with too much carbon potential can cause sooting, while one with too much water vapor or CO2 can become decarburizing, even if it's oxygen-free.
Making the Right Choice for Your Goal
The choice between an air furnace and a specific controlled atmosphere depends entirely on the material, the process, and the final requirements for the component.
- If your primary focus is simple stress relief or tempering at low temperatures: An open-air furnace is often sufficient, as the rate of oxidation is minimal and acceptable.
- If your primary focus is achieving a scale-free, bright finish after annealing: An inert (Nitrogen) or reducing (dissociated ammonia, hydrogen) atmosphere is necessary.
- If your primary focus is hardening a steel part without surface degradation: A neutral-to-reducing atmosphere (Endothermic gas) is required to prevent decarburization and maintain surface carbon.
- If your primary focus is increasing surface hardness and wear resistance: An active carburizing atmosphere is the definitive method for case hardening steel components.
By precisely managing the gaseous environment, you transition from simply heating metal to truly engineering its final properties and surface integrity.
Summary Table:
| Goal | Recommended Atmosphere Type | Key Benefit |
|---|---|---|
| Scale-free, bright finish | Inert (Nitrogen/Argon) or Reducing (Hydrogen) | Prevents oxidation, maintains surface finish |
| Hardening without surface degradation | Neutral-to-Reducing (Endothermic Gas) | Prevents decarburization, maintains surface carbon |
| Increase surface hardness (Case Hardening) | Active Carburizing Atmosphere | Diffuses carbon into the surface for a hard, wear-resistant case |
Achieve flawless metallurgical results with KINTEK's advanced controlled atmosphere furnaces.
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements.
Ready to eliminate oxidation and decarburization from your heat treatment process? Contact our experts today to discuss how our tailored furnace solutions can protect your components and ensure superior surface integrity.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process



















