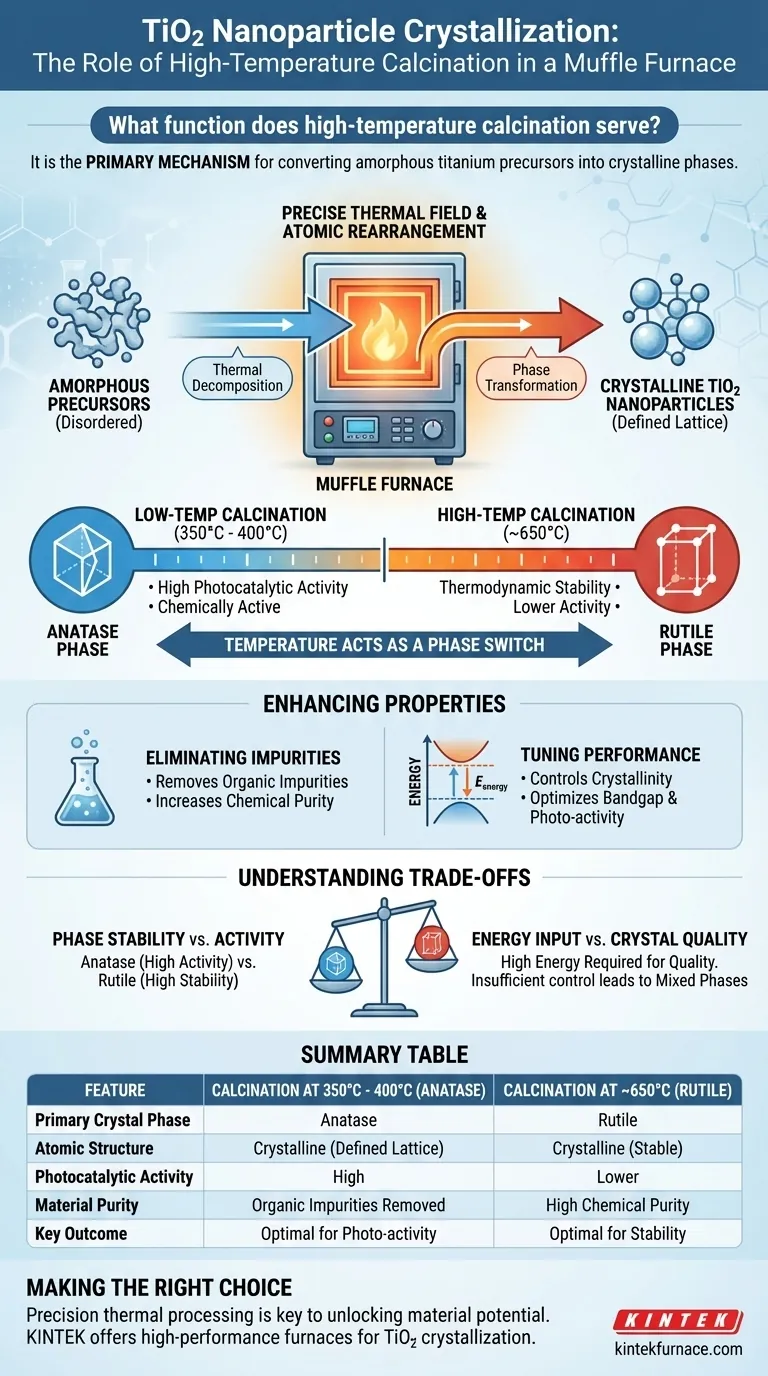
High-temperature calcination serves as the primary mechanism for converting amorphous titanium precursors into specific, crystalline phases of titanium dioxide (TiO2). By subjecting the material to a precise temperature field within a muffle furnace, the process facilitates thermal decomposition and atomic rearrangement. This transforms disordered precipitates into targeted crystal structures, such as anatase or rutile, directly determining the nanoparticle's final physical and chemical properties.
Calcination is not merely a drying process; it is a precise phase-selection tool that dictates the material's identity. By manipulating the furnace temperature, you directly control the crystal structure, purity, and photocatalytic potential of the final TiO2 nanoparticles.

Driving Phase Transformation
From Amorphous to Crystalline
The initial precipitates of titanium salts are typically amorphous, lacking a defined long-range atomic order.
Calcination provides the thermal energy required to reorganize these atoms. This creates a stable, repeating lattice structure, effectively turning the raw precursor into functional TiO2 nanoparticles.
Temperature as a Phase Switch
The specific temperature set within the muffle furnace acts as a switch between different crystal phases.
According to experimental data, calcining at approximately 350°C to 400°C typically yields the anatase phase. Conversely, raising the temperature to 650°C drives the transformation into the rutile phase.
The Role of the Muffle Furnace
A muffle furnace is essential because it provides a highly stable thermal environment.
This stability ensures that the thermal decomposition occurs uniformly throughout the sample. Precise control of the temperature field prevents uneven crystallization, ensuring the entire batch achieves the desired phase.
Enhancing Material Properties
Eliminating Impurities
Beyond crystallization, the high-temperature environment serves a critical purification function.
It effectively eliminates organic impurities remaining from the synthesis process. This results in a chemically pure material with higher adsorption activity.
Tuning Performance
The degree of crystallinity achieved during calcination directly influences the material's bandgap energy.
By controlling the temperature, you can fine-tune the electronic properties of the nanoparticles. This optimization is vital for maximizing the material's photocatalytic activity.
Understanding the Trade-offs
Phase Stability vs. Activity
While higher temperatures generally increase the degree of crystallinity, "more heat" is not always better.
The anatase phase (formed at lower temperatures) is often more chemically active for certain applications. Pushing the temperature too high (e.g., toward 650°C) forces a transition to rutile, which is more thermodynamically stable but may have different optical and catalytic behaviors.
Energy Input vs. Crystal Quality
Achieving high crystallinity requires significant thermal energy input.
However, insufficient temperature control can lead to a mix of phases (e.g., a blend of anatase and rutile). This lack of phase purity can degrade the specific performance characteristics required for advanced applications.
Making the Right Choice for Your Goal
To optimize your TiO2 synthesis, you must align your furnace settings with your specific application requirements.
- If your primary focus is Photocatalytic Activity (Anatase): Target a calcination temperature range of 350°C to 400°C to maximize the formation of the anatase phase while ensuring organic impurities are removed.
- If your primary focus is Thermodynamic Stability (Rutile): Increase the calcination temperature to roughly 650°C to drive the complete transformation into the rutile phase.
Precise thermal management is the difference between a generic powder and a high-performance nanomaterial.
Summary Table:
| Feature | Calcination at 350°C - 400°C | Calcination at ~650°C |
|---|---|---|
| Primary Crystal Phase | Anatase | Rutile |
| Atomic Structure | Crystalline (Defined Lattice) | Crystalline (Thermodynamically Stable) |
| Photocatalytic Activity | High | Lower |
| Material Purity | Organic Impurities Removed | High Chemical Purity |
| Key Outcome | Optimal for Photo-activity | Optimal for Stability |
Precision thermal processing is the key to unlocking the full potential of your nanomaterials. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of TiO2 crystallization. Whether you need an Anatase-specific profile or a high-temperature Rutile transition, our customizable lab high-temp furnaces provide the stability and control your research requires. Contact KINTEK today to discuss your unique synthesis needs and elevate your material quality.
Visual Guide
References
- A. C. W. W. M. N. Peshala Koswatta, Atula S. D. Sandanayaka. Boosting Solar Cell Efficiency: Enhancing Dye-Sensitized Solar Cell Performance with Carbon Quantum Dots and Titanium Dioxide Nanostructures from Sri Lankan Ilmenite. DOI: 10.1021/acsomega.5c02272
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How do you prevent maintenance on a muffle furnace? Extend Lifespan with Proactive Care
- How to calibrate a muffle furnace? Ensure Precise Temperature Control for Your Lab
- What atmosphere control options are available in advanced muffle furnaces? Master Materials Processing with Precision
- Why is a high-temperature muffle furnace used for Ni-BN powder preheating? Achieve defect-free coating density.
- What is the conclusion regarding the use of muffle furnaces? Essential for Clean, High-Temp Processing
- What are the construction details of a typical muffle furnace? Key Components for High-Temperature Control
- How is a laboratory high-temperature muffle furnace utilized in g-C3N4 synthesis? Optimize Your Thermal Polycondensation
- What role does a high-temperature muffle furnace play in the electrodeposition of high-purity iron? Achieve Precision



















