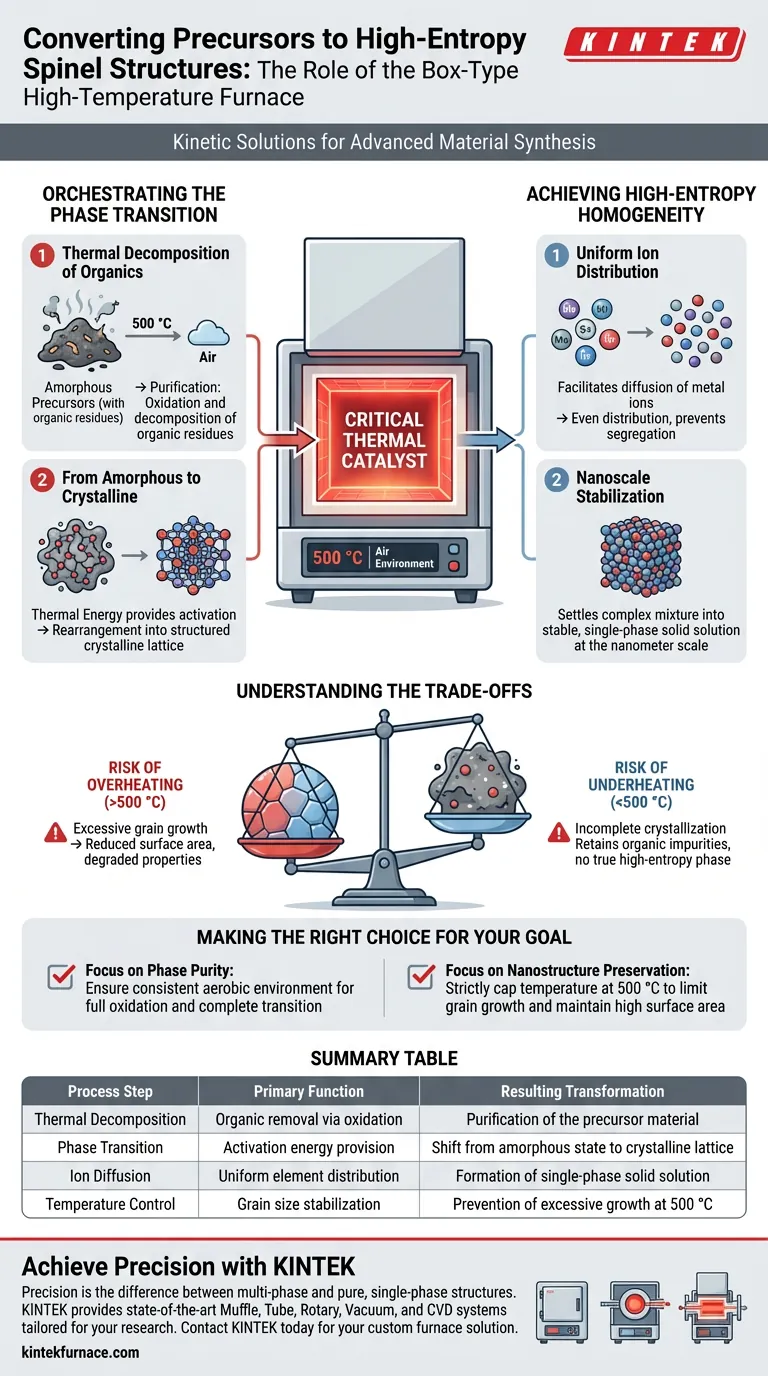
The box-type high-temperature furnace acts as the critical thermal catalyst for transforming amorphous precursors into ordered, single-phase high-entropy spinel structures. By maintaining a constant, controlled environment of 500 °C in air, the furnace facilitates the thermal decomposition of residual organic components. Simultaneously, it provides the precise kinetic energy required to crystallize the material and induce a uniform distribution of metal ions at the nanometer scale.
Core Insight: The furnace’s primary value lies in its ability to balance crystallization with grain size control. It provides enough heat to form the complex single-phase structure but maintains a low enough temperature (500 °C) to prevent the excessive grain growth that typically degrades material performance at higher ranges.

Orchestrating the Phase Transition
Thermal Decomposition of Organics
The initial function of the furnace is purification. The precursor materials often contain organic residues from the synthesis stage.
The 500 °C air environment ensures these residual components are effectively removed through oxidation and decomposition.
From Amorphous to Crystalline
Before entering the furnace, the precursors exist in an amorphous (disordered) state.
The furnace provides the thermal energy necessary to overcome the activation energy barrier, rearranging the atoms into a structured, crystalline lattice.
This transition is essential for establishing the specific geometry of the spinel structure.
Achieving High-Entropy Homogeneity
Uniform Ion Distribution
High-entropy materials consist of five or more elements that must be mixed randomly but uniformly within the crystal lattice.
The furnace facilitates the diffusion of metal ions, ensuring they are distributed evenly throughout the structure rather than segregating into clumps.
Nanoscale Stabilization
This process happens at the nanometer scale. The thermal treatment ensures that the complex mixture of elements settles into a stable, single-phase solid solution.
Without this controlled heating, the material might separate into multiple, unwanted phases rather than a single cohesive spinel structure.
Understanding the Trade-offs
The Risk of Overheating
While heat is necessary for formation, "more" is not always "better" in this specific application.
If the furnace temperature significantly exceeds 500 °C, the individual crystalline grains will begin to merge and grow larger.
This excessive grain growth reduces the surface area and can negatively impact the unique properties derived from the nanostructure.
The Risk of Underheating
Conversely, failing to maintain the 500 °C threshold may result in incomplete crystallization.
This leaves the material partially amorphous or retaining organic impurities, which prevents the formation of a true high-entropy spinel phase.
Making the Right Choice for Your Goal
When configuring your thermal treatment for high-entropy spinel structures, consider your specific objectives:
- If your primary focus is Phase Purity: Ensure the furnace maintains a consistent aerobic environment to fully oxidize organic residuals and complete the amorphous-to-crystalline transition.
- If your primary focus is Nanostructure Preservation: Strictly cap your temperature at 500 °C to strictly limit grain growth and maintain high surface area.
Success depends on utilizing the furnace not just as a heater, but as a precision tool for controlling the kinetics of atomic arrangement.
Summary Table:
| Process Step | Primary Function | Resulting Transformation |
|---|---|---|
| Thermal Decomposition | Organic removal via oxidation | Purification of the precursor material |
| Phase Transition | Activation energy provision | Shift from amorphous state to crystalline lattice |
| Ion Diffusion | Uniform element distribution | Formation of single-phase solid solution |
| Temperature Control | Grain size stabilization | Prevention of excessive growth at 500 °C |
Achieve Precision in Advanced Material Synthesis with KINTEK
Precision is the difference between a multi-phase mixture and a pure, single-phase high-entropy structure. KINTEK provides state-of-the-art thermal solutions tailored for complex materials research. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific research temperatures and atmospheric requirements.
Whether you are focusing on nanostructure preservation or large-scale phase purity, our lab high-temperature furnaces deliver the thermal stability you need. Contact KINTEK today to find your custom furnace solution and elevate your material performance.
Visual Guide
References
- Ayano Taniguchi, Kazuya Kobiro. Low-temperature synthesis of porous high-entropy (CoCrFeMnNi)<sub>3</sub>O<sub>4</sub> spheres and their application to the reverse water–gas shift reaction as catalysts. DOI: 10.1039/d3dt04131j
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why are muffle furnaces particularly useful in material science? Unlock Precise, Contaminant-Free Heat Treatment
- Why is a laboratory oven required for synthesis of doped Nickel Oxide nanopowders? Ensure Material Structural Integrity
- What is the function of a high-temperature box furnace in Cu-Ni-P alloy annealing? Optimize Your Cold Rolling Results
- What materials are commonly used in the construction of a muffle furnace? Discover Durable Components for High-Temp Labs
- How does a box-type high-temperature furnace contribute to 6Mo stainless steel? Optimize Solution Treatment Now
- What makes crucible furnaces suitable for high-temperature applications? Achieve Unmatched Purity and Precision
- Why is a box-type resistance furnace utilized for long-duration heat preservation of chromium steel? Key Benefits
- What are the common applications of a muffle furnace? Unlock Precise Heat Treatment for Your Lab



















