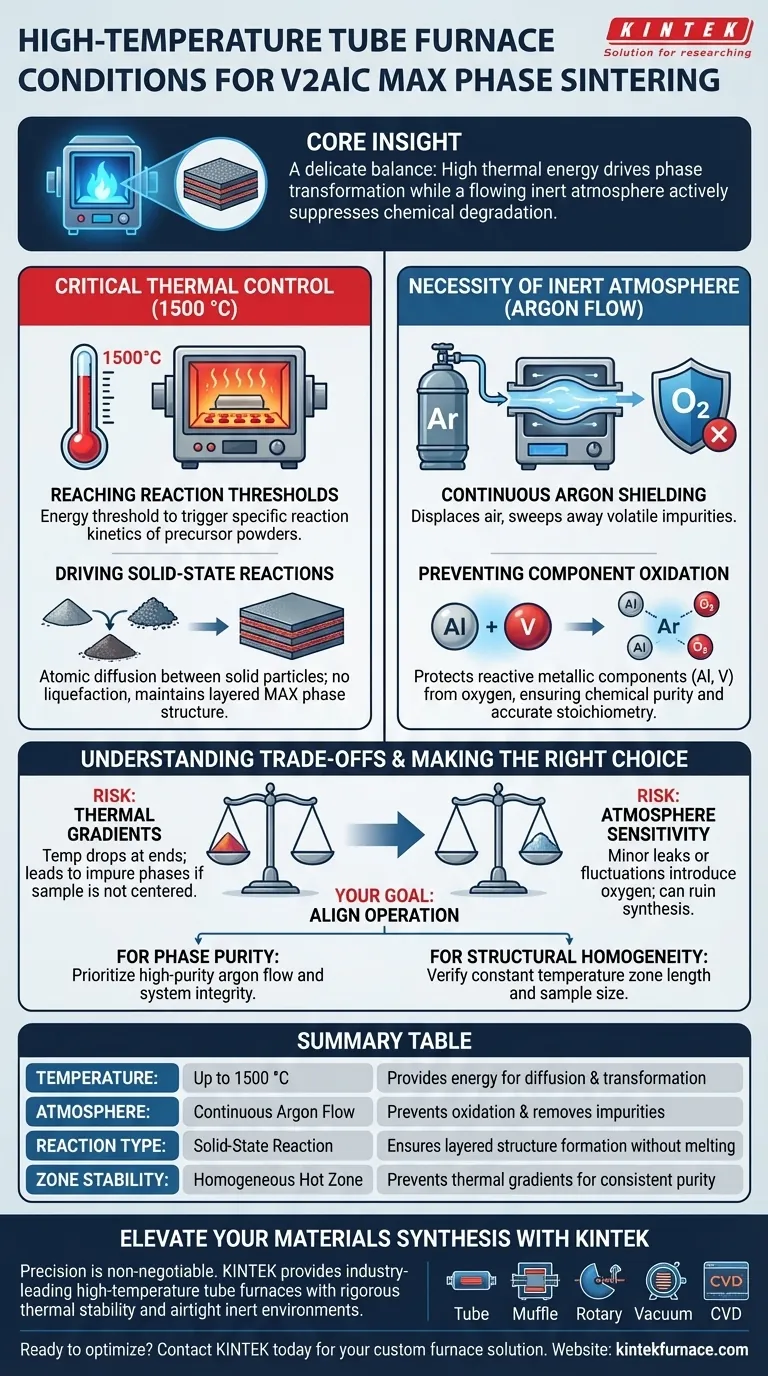
A high-temperature tube furnace establishes a strictly controlled environment characterized by extreme heat, reaching up to 1500 °C, and a continuous flow of inert argon gas. This specific combination is engineered to facilitate the solid-state reaction necessary to synthesize V2AlC MAX phase materials while rigorously protecting the reactive metallic components from oxidation.
Core Insight: The synthesis of V2AlC is not just about heating powders; it requires a delicate balance where high thermal energy drives phase transformation while a flowing inert atmosphere actively suppresses the chemical degradation of the material.

The Critical Role of Thermal Control
Reaching Reaction Thresholds
To successfully synthesize V2AlC, the furnace must provide a stable high-temperature zone capable of reaching 1500 °C.
This extreme heat is not arbitrary; it is the energy threshold required to trigger the specific reaction kinetics of the precursor powders.
Driving Solid-State Reactions
The tube furnace environment ensures that the precursor powders undergo a solid-state reaction.
Unlike melting, this process relies on atomic diffusion between solid particles. The precise thermal control of the furnace maintains the material at the exact temperature needed for these atoms to rearrange into the layered MAX phase structure without liquefying.
The Necessity of an Inert Atmosphere
Continuous Argon Shielding
A static atmosphere is often insufficient for MAX phase synthesis; the furnace provides a continuous flow of inert argon gas.
This dynamic flow serves two purposes: it displaces any existing air within the tube and constantly sweeps away volatile impurities that might be released during heating.
Preventing Component Oxidation
The primary threat to V2AlC synthesis is oxygen, as metallic components like aluminum and vanadium are highly susceptible to oxidation at elevated temperatures.
By maintaining an oxygen-free environment, the furnace prevents the formation of unwanted oxides. This ensures the chemical purity of the final product and guarantees that the stoichiometry of the V2AlC phase remains accurate.
Understanding the Trade-offs
The Risk of Thermal Gradients
While tube furnaces offer precise control, they can sometimes exhibit thermal gradients where the temperature drops toward the ends of the tube.
If the sample is not positioned perfectly in the center of the "hot zone," the solid-state reaction may be incomplete, leading to impure phases.
Atmosphere Sensitivity
The system relies entirely on the integrity of the inert gas flow.
Even a minor leak or a fluctuation in the argon flow rate can introduce enough oxygen to ruin the synthesis process at 1500 °C. The dependence on high-purity gas adds an operational cost and a critical failure point that must be monitored.
Making the Right Choice for Your Goal
To maximize the quality of your V2AlC synthesis, align your furnace operation with your specific objectives:
- If your primary focus is Phase Purity: Prioritize the integrity of the gas delivery system and ensure high-purity argon is flowing before heating begins to flush out all contaminants.
- If your primary focus is Structural Homogeneity: Verify the length of the furnace's constant temperature zone and ensure your sample boat is small enough to fit entirely within this uniform region.
Ultimately, the quality of your V2AlC material depends as much on the exclusion of oxygen as it does on the application of heat.
Summary Table:
| Feature | Requirement for V2AlC Sintering | Role in the Process |
|---|---|---|
| Temperature | Up to 1500 °C | Provides energy for solid-state atomic diffusion and phase transformation. |
| Atmosphere | Continuous Argon Flow | Prevents oxidation of metallic components and sweeps away volatile impurities. |
| Reaction Type | Solid-State Reaction | Ensures layered MAX phase structure formation without liquefying the material. |
| Zone Stability | Homogeneous Hot Zone | Prevents thermal gradients to ensure consistent phase purity across the sample. |
Elevate Your Materials Synthesis with KINTEK
Precision is non-negotiable when synthesizing sensitive MAX phase materials like V2AlC. KINTEK provides industry-leading high-temperature tube furnaces designed to deliver the rigorous thermal stability and airtight inert environments your research demands.
Backed by expert R&D and world-class manufacturing, our systems—including Tube, Muffle, Rotary, Vacuum, and CVD furnaces—are fully customizable to meet your unique laboratory specifications. Don't let oxidation or thermal gradients compromise your results.
Ready to optimize your sintering process? Contact KINTEK today to discuss your custom furnace solution.
Visual Guide
References
- Reagan A. Beers, Jessica R. Ray. Chemical Intercalant Affects the Structural Properties and Aqueous Stability of V<sub>2</sub>CT<sub>x</sub> MXene. DOI: 10.1002/admi.202500145
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does a laboratory horizontal tube furnace facilitate the sintering of powder metallurgy structural steel?
- What is the significance of using a vacuum tube furnace for Fe3Al powder? Optimize Magnetic Phase Transformation
- How does a tube furnace achieve high thermal efficiency? Optimize Energy Use for Cost Savings
- What role does a high-temperature tube furnace play in biomass-derived carbon? Unlock Advanced Material Synthesis
- What are the benefits of independent temperature control in a three-zone furnace? Enhance Precision and Uniformity
- What is an alumina tube furnace? Essential for High-Temp, Contamination-Free Material Processing
- What are the controlled atmosphere capabilities of a tube furnace? Unlock Precise Gas Control for Your Lab
- What materials are used in tube furnace? Key Components for High-Temp Lab Success



















