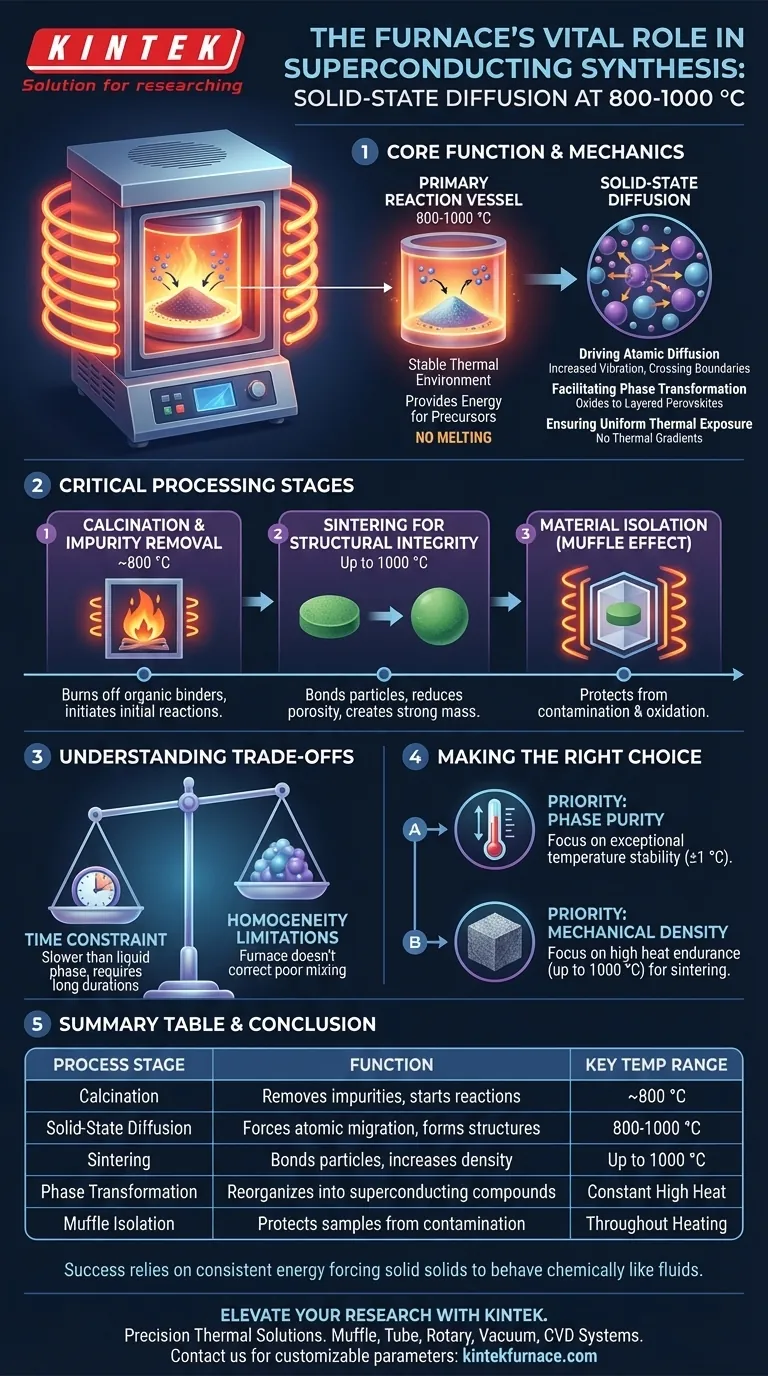
A high-temperature box resistance furnace acts as the primary reaction vessel for the solid-state synthesis of superconducting materials. It creates a stable thermal environment, typically maintained between 800 and 1000 °C, which provides the energy required for precursor powders to react chemically and structurally without melting.
The furnace’s primary role is to drive solid-state diffusion. By maintaining precise heat over long durations, it forces atoms to migrate between solid particles, rearranging them into the specific, layered crystal structures necessary for superconductivity.

The Mechanics of Solid-State Synthesis
Driving Atomic Diffusion
In solid-state synthesis, materials are not melted into a liquid to mix. Instead, the furnace provides enough thermal energy to increase atomic vibration, allowing atoms to physically move (diffuse) across the boundaries of powder particles.
Facilitating Phase Transformation
This diffusion triggers a chemical phase transformation. The mixture of raw precursor oxides changes fundamental states, reorganizing into complex superconducting compounds, such as layered perovskite structures.
Ensuring Uniform Thermal Exposure
The "box" or "muffle" design ensures that heat is applied uniformly from all sides. This uniformity is critical to prevent thermal gradients, which could lead to uneven reaction rates and impure sections within the final superconducting sample.
Critical Processing Stages
Calcination and Impurity Removal
Before the final structure is formed, the furnace is often used for pre-calcination (often around 800 °C). This step burns off organic binders or impurities and triggers the initial solid-state reactions between constituent oxides.
Sintering for Structural Integrity
Following calcination, the furnace performs sintering. This process heats the "green compact" (pressed powder) to bond the particles together, reducing porosity and creating a dense, mechanically strong solid mass.
Material Isolation (The "Muffle" Effect)
The design of a muffle furnace encloses the material in a separate chamber (the muffle). This protects the superconductor from direct contact with heating elements or combustion byproducts, preventing contamination and oxidation that would degrade electrical properties.
Understanding the Trade-offs
The Constraint of Time
Solid-state diffusion is inherently slower than liquid-phase reactions. Consequently, this synthesis method requires significant time inside the furnace to ensure the reaction permeates the entire volume of the material.
Homogeneity Limitations
While the furnace provides uniform heat, it cannot correct for poorly mixed precursor powders. If the initial physical mixing is inadequate, the limited range of atomic diffusion means the final superconductor will lack chemical homogeneity.
Making the Right Choice for Your Goal
If your primary focus is Phase Purity: Prioritize a furnace with exceptional temperature stability (±1 °C) to ensure the material stays strictly within the narrow window required for perovskite formation.
If your primary focus is Mechanical Density: Focus on the sintering capabilities of the unit, ensuring it can maintain high heat (up to 1000 °C) for extended durations to maximize particle bonding and reduce porosity.
The success of superconducting synthesis ultimately relies on the furnace's ability to deliver consistent energy that forces solid solids to behave chemically like fluids.
Summary Table:
| Process Stage | Function in Synthesis | Key Temperature Range |
|---|---|---|
| Calcination | Removes impurities and initiates precursor reactions | ~800 °C |
| Solid-State Diffusion | Forces atomic migration to form layered crystal structures | 800 - 1000 °C |
| Sintering | Bonds particles to increase density and structural integrity | Up to 1000 °C |
| Phase Transformation | Reorganizes oxides into complex superconducting compounds | Constant High Heat |
| Muffle Isolation | Protects samples from contamination and oxidation | Throughout Heating |
Elevate Your Superconducting Research with KINTEK
Precision is paramount when managing the delicate phase transformations required for superconducting materials. At KINTEK, we specialize in high-performance thermal solutions designed for rigorous lab environments. Backed by expert R&D and world-class manufacturing, we provide a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your specific synthesis parameters.
Whether you need exceptional temperature stability for phase purity or extended high-heat endurance for sintering, KINTEK furnaces deliver the uniform thermal environment your materials demand. Contact us today to discuss your unique needs and see how our advanced laboratory furnaces can accelerate your breakthroughs in material science.
Visual Guide
References
- T. Chattopadhyay. Superconductivity in High-Temperature Materials. DOI: 10.36948/ijfmr.2025.v07i05.55511
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is application important when selecting a muffle furnace? Ensure Optimal Performance for Your Lab
- What role does a high-temperature box furnace play in the pre-calcination of LLZTO? Master Garnet Phase Synthesis
- What are the advantages of using a Microwave Muffle Furnace? Faster, Higher-Quality Activated Carbon Preparation
- What factors should be considered before buying a muffle furnace? Ensure Safety and Efficiency for Your Lab
- What role does a muffle furnace play in the calcination of metal catalysts? Optimize Thermal Stability and Reactivity
- What are the specific uses of muffle furnaces in laboratories? Essential for Contaminant-Free High-Temp Processes
- Why is a programmable temperature control furnace necessary for sintering HA? Ensure Structural Integrity & Purity
- What is the primary function of a high-temperature box resistance furnace in HA synthesis? Optimize Your Calcination.



















