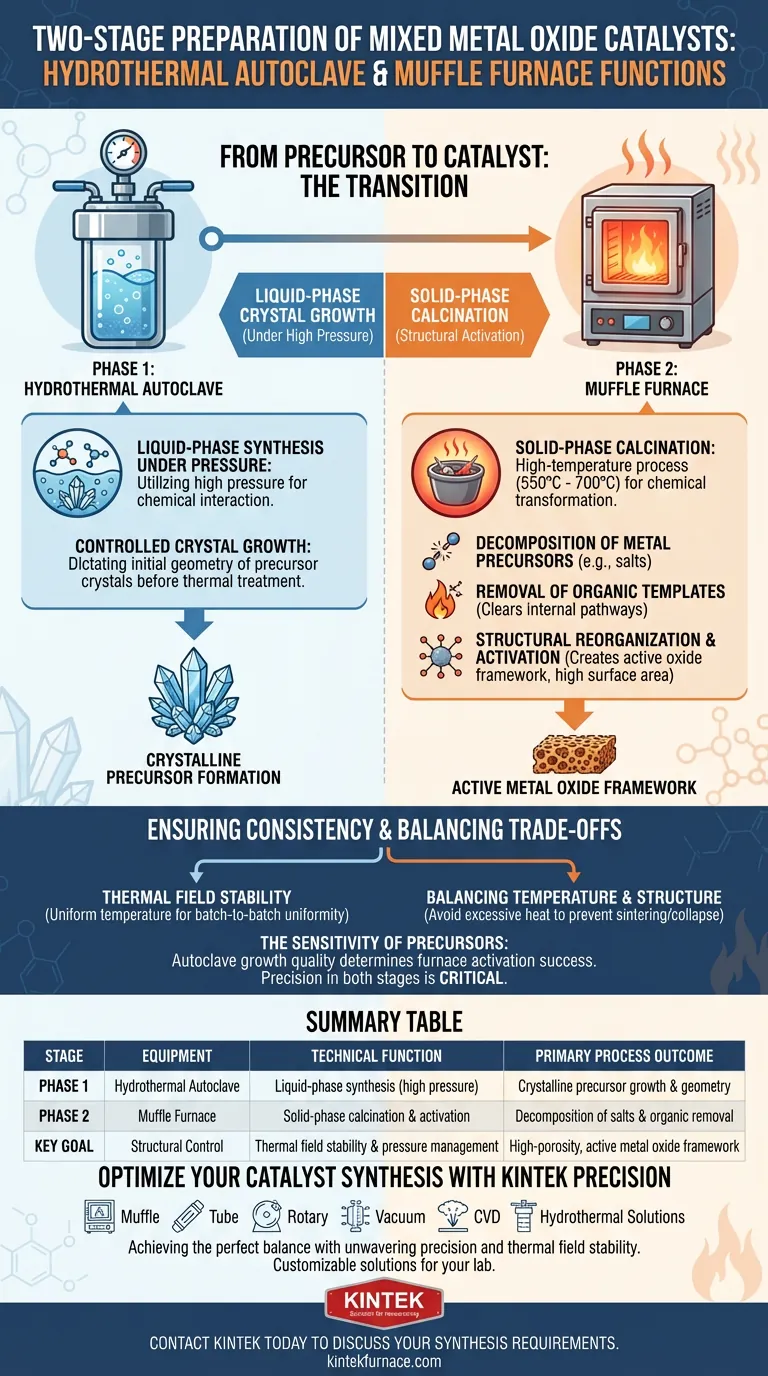
In the two-stage preparation of mixed metal oxide catalysts, the hydrothermal autoclave and the muffle furnace serve two opposing but complementary technical functions: the autoclave facilitates liquid-phase crystal growth under high pressure, while the muffle furnace performs solid-phase calcination to activate the structure.
Core Takeaway The hydrothermal autoclave constructs the initial crystalline architecture in a pressurized liquid environment, while the muffle furnace locks in this structure and chemically activates it. This transition—from growing a precursor to calcining a final product—removes organic barriers and converts inert salts into a porous, catalytically active oxide framework.
Phase 1: The Hydrothermal Autoclave
Liquid-Phase Synthesis Under Pressure
The primary function of the hydrothermal autoclave is to create an environment for liquid-phase synthesis that standard laboratory glassware cannot support. By utilizing high pressure, it forces the interaction of chemical components to form crystalline precursors.
Controlled Crystal Growth
Within the sealed environment of the autoclave, temperature and pressure work together to enable the specific growth of precursor crystals. This stage dictates the initial geometry and fundamental arrangement of the metal ions before any high-temperature thermal treatment occurs.
Phase 2: The Muffle Furnace
Solid-Phase Calcination
Once the precursor is dried, the muffle furnace takes over for the critical calcination stage. This is a high-temperature process (typically ranging from 550°C to 700°C) designed to chemically transform the material from a solid precursor into a functional catalyst.
Decomposition of Metal Precursors
The furnace provides the thermal energy required to decompose metal salt precursors. Compounds such as nickel acetate or copper nitrate are chemically broken down and converted into their active metal oxide forms.
Removal of Organic Templates
To create a high specific surface area, catalysts often use surfactant templates during synthesis. The muffle furnace burns off these residual organic components. This elimination clears the internal pathways, exposing the pores necessary for catalytic reactions.
Structural Reorganization and Activation
Beyond simple drying, the furnace facilitates a reorganization of the inorganic framework. This heat treatment generates specific active centers and facilitates chemical transformations, such as the creation of magnetic nickel-ferrite (NiFe2O4) components. It activates carriers (like activated carbon) to create hollow porous structures, significantly improving the material's ability to contact and activate reactants.
Ensuring Process Consistency
Thermal Field Stability
A specific advantage of a high-quality laboratory muffle furnace is its thermal field stability. In catalyst preparation, slight deviations in temperature can alter the skeletal structure of the oxide.
Batch-to-Batch Uniformity
The furnace ensures that the temperature curve is applied uniformly across the sample. This stability is the key factor in ensuring that different batches of catalysts possess consistent physical properties and catalytic performance.
Understanding the Trade-offs
Balancing Temperature and Structure
While high temperatures are necessary for activation, they present a critical trade-off. The muffle furnace must reach temperatures high enough to fully decompose salts and remove surfactants (e.g., 550°C for 6 hours). However, excessive heat or uncontrolled duration can lead to the collapse of the porous structure or "sintering," which reduces the active surface area.
The Sensitivity of Precursors
The autoclave stage creates a precursor that is sensitive to the subsequent heat treatment. If the crystalline growth in the autoclave is insufficient, the furnace cannot "fix" the structure. Conversely, a perfect precursor can be ruined by an unstable thermal field in the furnace, emphasizing the need for precision in both stages.
Making the Right Choice for Your Goal
To optimize your mixed metal oxide catalyst, focus on the specific parameters of each stage according to your desired outcome:
- If your primary focus is defining the initial crystal geometry: Concentrate on optimizing the pressure and time parameters of the hydrothermal autoclave to ensure robust precursor growth.
- If your primary focus is maximizing surface area and porosity: Prioritize the muffle furnace ramp rates and hold times to ensure complete removal of surfactants without collapsing the skeletal structure.
- If your primary focus is reproducible industrial performance: Ensure your muffle furnace has verified thermal field stability to guarantee that every batch undergoes identical chemical decomposition.
Mastering the transition from the pressurized growth of the autoclave to the thermal activation of the furnace is the defining factor in high-performance catalyst synthesis.
Summary Table:
| Stage | Equipment | Technical Function | Primary Process Outcome |
|---|---|---|---|
| Phase 1 | Hydrothermal Autoclave | Liquid-phase synthesis under high pressure | Crystalline precursor growth and geometry |
| Phase 2 | Muffle Furnace | Solid-phase calcination and activation | Decomposition of salts and organic removal |
| Key Goal | Structural Control | Thermal field stability & pressure management | High-porosity, active metal oxide framework |
Optimize Your Catalyst Synthesis with KINTEK Precision
Achieving the perfect balance between precursor growth and thermal activation requires equipment that delivers unwavering precision. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized hydrothermal solutions tailored for your laboratory needs.
Whether you are refining crystal geometry or maximizing specific surface area, our customizable high-temp furnaces ensure the thermal field stability essential for reproducible, high-performance catalysts.
Ready to elevate your material research? Contact KINTEK today to discuss your unique synthesis requirements!
Visual Guide
References
- Zi‐Qing Liu, Bao‐Li Fei. Mixed Metal Oxide Derived from Polyoxometalate-Based Metal–Organic Framework as a Bi-Functional Heterogeneous Catalyst for Wastewater Treatment. DOI: 10.3390/catal15010076
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What advantages do muffle furnaces offer for material processing? Achieve Precision and Purity in Heat Treatment
- Why is temperature stability important in a muffle furnace? Ensure Reliable Results and Process Control
- What role does a muffle furnace play in the preparation of Cu-MnOx/GF catalytic electrodes? | Enhanced Synthesis Guide
- What is the role of a high-temperature muffle furnace in PNCO-impregnated electrode post-treatment? Master Sintering
- What role does a high-temperature box furnace play in the pre-calcination of LLZTO? Master Garnet Phase Synthesis
- Why is a programmable temperature ramp rate essential in muffle furnace operations for nanomaterial precursors?
- What is the specific role of a box muffle furnace in the austenitizing of Vanadis 60? Achieve Precise Hardening Control
- How does a muffle furnace ensure contamination-free heating? Discover Its Isolation Design for Purity



















