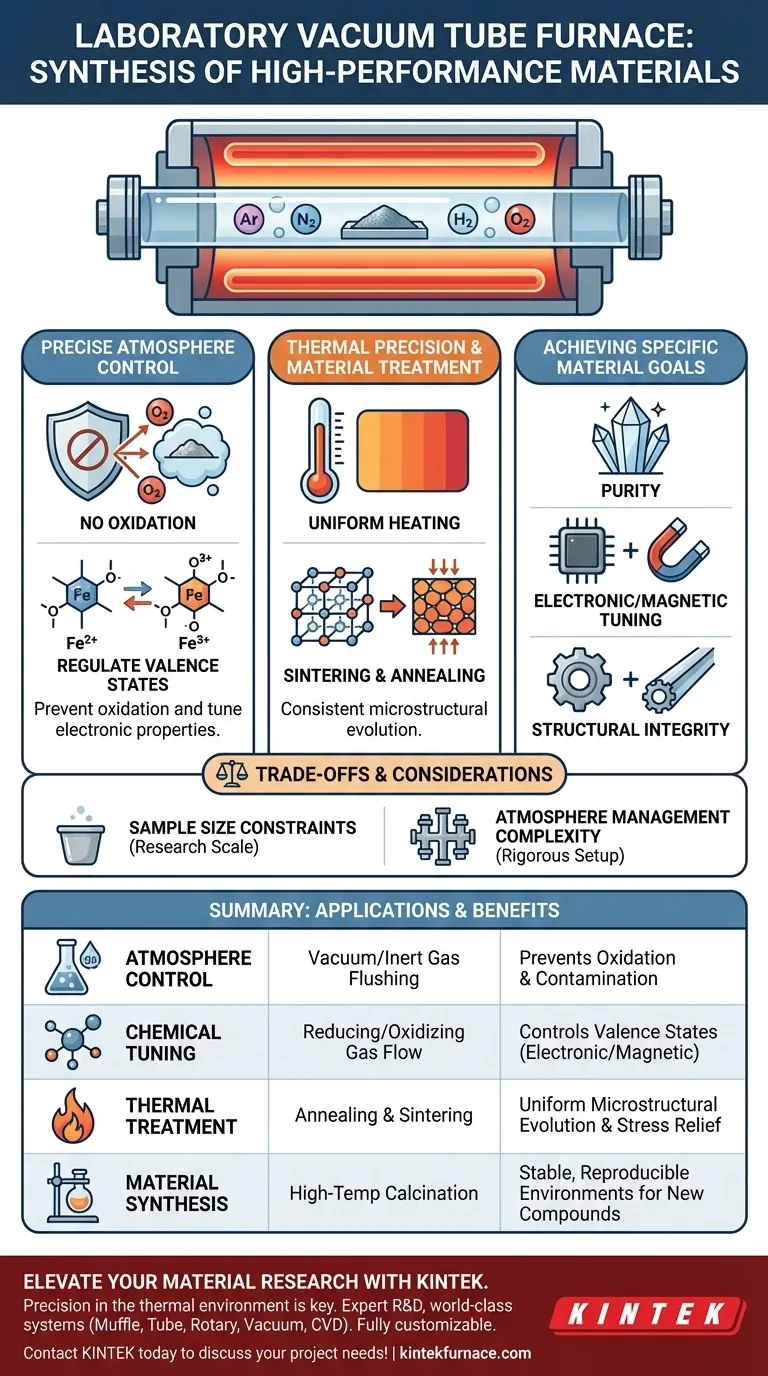
Laboratory vacuum tube furnaces are fundamental tools in the synthesis of high-performance chemical materials, primarily utilized to create precisely controlled thermal and atmospheric environments. They are specifically applied to perform high-temperature annealing and calcination under vacuum, inert, reducing, or oxidizing conditions. This capability allows researchers to strictly prevent sample oxidation and manipulate the chemical valence states of active components to meet exact specifications.
By isolating materials from uncontrolled ambient air, these furnaces provide the contamination-free environment necessary to study microstructural evolution and optimize the mechanical and chemical properties of advanced compounds.

Controlling the Chemical Environment
The most critical application of a vacuum tube furnace is its ability to dictate the gas atmosphere surrounding a sample.
Preventing Oxidation
In high-performance synthesis, even trace amounts of oxygen can degrade a material's properties. A vacuum tube furnace effectively removes air to create a vacuum or introduces inert gases (like argon or nitrogen). This prevents the unwanted oxidation of sensitive samples during heating.
Regulating Valence States
Beyond simple protection, these furnaces actively shape the chemical structure of the material. By introducing reducing or oxidizing gases into the tube, researchers can control the chemical valence states of active components. This precision is essential for synthesizing materials where specific electronic or magnetic properties are required.
Thermal Precision and Material Treatment
High-performance materials require more than just heat; they require thermal uniformity and stability.
Uniform Heating
The furnace ensures a highly uniform temperature field along the length of the tube. This prevents thermal gradients that could lead to inconsistent material properties across a single sample. Uniformity is vital for reproducible results in both research and small-scale production.
Phase Transformation and Sintering
Researchers use these furnaces to study and induce phase transformations and microstructural evolution. Processes such as sintering and annealing are used to densify materials and relieve internal stresses. This results in superior mechanical properties, crucial for superalloys and advanced ceramics.
Understanding the Trade-offs
While vacuum tube furnaces offer exceptional control, there are inherent limitations to consider regarding throughput and scale.
Sample Size Constraints
Tube furnaces are generally designed for heating small samples. They are ideal for research and synthesis of inorganic compounds but are rarely suitable for bulk industrial processing. If you require large-volume production, a different furnace architecture may be necessary.
Complexity of Atmosphere Management
Achieving the "perfect" environment requires rigorous setup. Maintaining a high vacuum or managing precise gas flow rates adds complexity to the experimental design. Leaks or pump failures can compromise the entire synthesis process, requiring constant vigilance.
Making the Right Choice for Your Goal
To maximize the utility of a laboratory vacuum tube furnace, align your experimental setup with your specific material objectives.
- If your primary focus is purity: Prioritize high-vacuum capabilities to eliminate contaminants and prevent oxidation of reactive elements.
- If your primary focus is electronic property tuning: Utilize controlled gas flows (reducing or oxidizing) to precisely dictate the valence states of your active components.
- If your primary focus is structural integrity: Leverage the uniform temperature field to perform annealing or sintering for consistent microstructural evolution.
Precision in the environment leads to precision in the material.
Summary Table:
| Application Category | Specific Process | Primary Benefit |
|---|---|---|
| Atmosphere Control | Vacuum/Inert Gas Flushing | Prevents oxidation and contamination of sensitive samples. |
| Chemical Tuning | Reducing/Oxidizing Gas Flow | Controls chemical valence states for electronic/magnetic properties. |
| Thermal Treatment | Annealing & Sintering | Ensures uniform microstructural evolution and stress relief. |
| Material Synthesis | High-Temp Calcination | Provides stable, reproducible environments for new compounds. |
Elevate Your Material Research with KINTEK
Precision in the thermal environment is the key to breakthrough material performance. Backed by expert R&D and world-class manufacturing, KINTEK provides a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need to prevent oxidation in superalloys or tune the electronic properties of advanced ceramics, our laboratory high-temp furnaces are fully customizable to meet your unique research specifications.
Ready to achieve superior thermal precision? Contact KINTEK today to discuss your project needs!
Visual Guide
References
- Bhupendra Pratap Singh, Rajendra Srivastava. Catalytic Hydrogenation of Lignin Ethers and Bio‐Oil Using Non‐Noble Cobalt Catalysts. DOI: 10.1002/cssc.202402714
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What precautions should be taken regarding liquids and metals in a tube furnace? Ensure Safety and Prevent Damage
- What recent advancements have been made in 70mm tube furnace technology? Discover Precision and Automation Innovations
- What are the specific requirements for quartz tubes used in fixed-bed reactors? Optimize Your CeAlOx/Ni-Foam Performance
- What factors should be considered when purchasing a drop tube furnace? Key specs for precision and efficiency
- What role does a tube annealing furnace play in the preparation of nanoporous NiPt catalysts? Vital Catalyst Activation
- What are the critical functions of a laboratory tube furnace in biomass synthesis? Optimize Your Carbonization Process
- How do vacuum tubes work for dummies? The Simple Analogy to Understand Electronic Control
- What is the basic function of a High Temperature Tube Furnace? Precision Thermal Processing for Material Synthesis



















