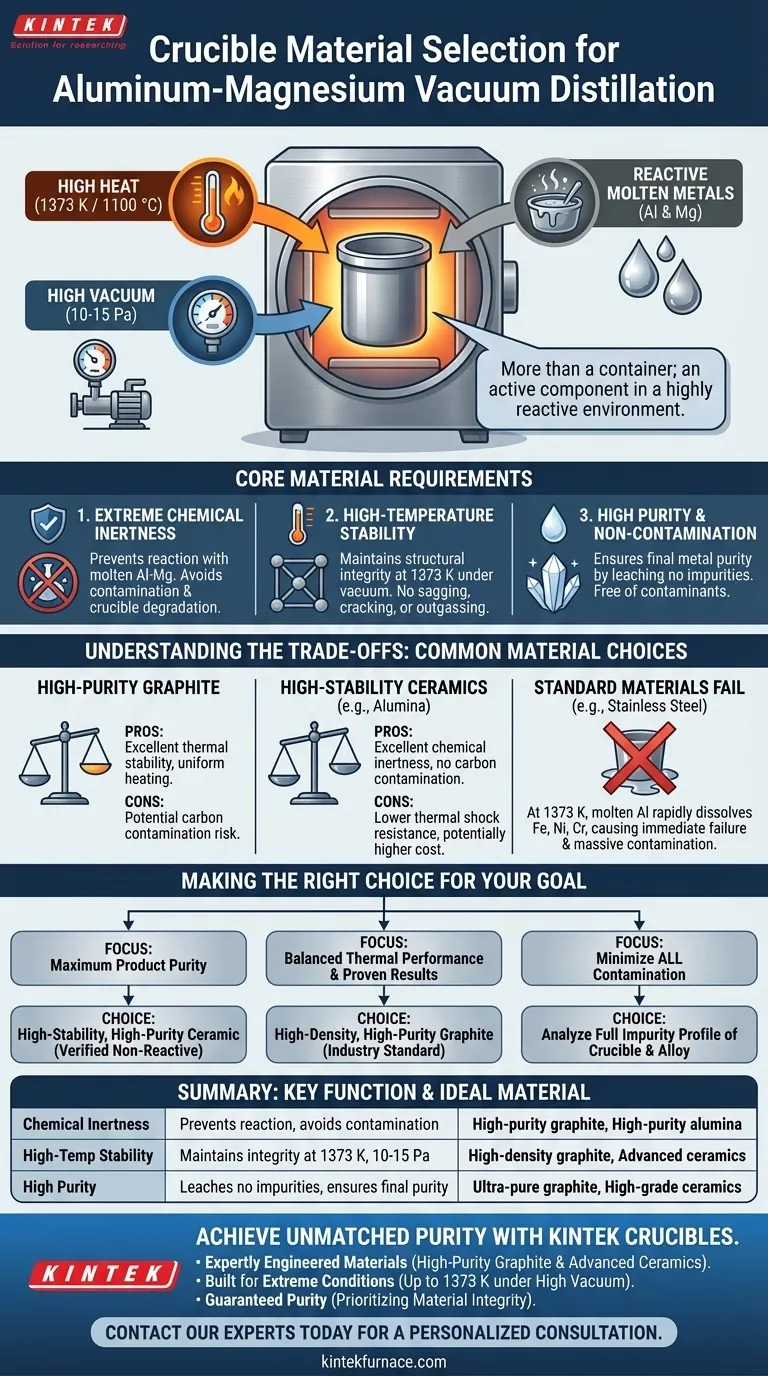
Crucible material selection is the foundational decision for successfully refining aluminum-magnesium alloys via vacuum distillation. The essential requirements are extreme chemical inertness to avoid reacting with the molten metals, high-temperature stability to withstand process conditions around 1373 K (1100 °C), and exceptional purity to prevent contamination of the final products.
The crucible is not merely a container; it is an active component in a highly reactive environment. The material chosen must survive the simultaneous assault of high heat, high vacuum, and chemically aggressive molten metals to ensure the integrity and purity of the separated aluminum and magnesium.

The Demanding Environment of Vacuum Distillation
To understand the material requirements, one must first appreciate the harsh conditions inside the vacuum furnace. The process is engineered to exploit the different boiling points of aluminum and magnesium, but this creates a hostile environment for any containment material.
The Role of High Temperature
The process operates at elevated temperatures, typically around 1373 K (1100 °C). This is necessary to significantly increase the vapor pressure of magnesium, allowing it to "boil" out of the liquid aluminum alloy. The crucible must maintain its structural integrity without melting, softening, or deforming at these temperatures.
The Challenge of High Vacuum
A high vacuum of 10-15 Pa is maintained within the furnace. This vacuum lowers the effective boiling point of magnesium and clears the path for its vapor to travel to a condensation surface. However, this environment can also accelerate the breakdown or outgassing of less-stable crucible materials.
The Reactivity of Molten Metals
Both molten aluminum and magnesium are highly reactive. They will readily attack, dissolve, or form compounds with many materials, especially at high temperatures. A crucible that reacts with the melt will not only be destroyed but will also fundamentally contaminate the very metals being purified.
Core Material Requirements Explained
The combination of these three factors—heat, vacuum, and chemical reactivity—dictates a very specific set of requirements for any suitable crucible material.
1. Extreme Chemical Inertness
This is the single most critical property. The crucible must be thermodynamically stable in the presence of molten Al-Mg alloy. Any reaction leads to two catastrophic failures: contamination of the high-purity metals and degradation of the crucible itself.
2. High-Temperature Stability
The material must possess a high melting point and low vapor pressure, ensuring it remains a solid, stable container throughout the process. It cannot sag, crack, or release any volatile components under the combined stress of heat and vacuum.
3. High Purity and Non-Contamination
The crucible itself must be free of impurities that could leach into the molten alloy. The goal of the process is to produce high-purity metals, and a crucible that introduces contaminants defeats the entire purpose.
Understanding the Trade-offs: Common Material Choices
No single material is perfect for every application. The choice often involves balancing performance, cost, and the specific purity requirements of the final product.
High-Purity Graphite
Graphite is a common choice due to its excellent thermal stability and good thermal conductivity, which promotes uniform heating. However, the primary risk is potential carbon contamination if the grade is not sufficiently pure or if the conditions favor carbide formation with the alloy.
High-Stability Ceramics
Advanced ceramics, such as high-purity alumina (corundum), are used when carbon contamination is unacceptable. They offer excellent chemical inertness against many metals. The main trade-off is often lower thermal shock resistance and potentially higher cost compared to graphite.
Why Standard Materials Fail
Materials like stainless steel are completely unsuitable for this high-purity application. At 1373 K, the molten aluminum would rapidly dissolve the iron, nickel, and chromium from the steel, leading to immediate crucible failure and massive contamination of the alloy.
Making the Right Choice for Your Goal
Selecting the correct crucible material is a strategic decision that directly impacts process efficiency and final product quality. Base your choice on the primary objective of your operation.
- If your primary focus is maximum product purity and avoiding carbon: A high-stability, high-purity ceramic crucible is the superior choice, provided it is verified to be non-reactive with your specific alloy.
- If your primary focus is balancing thermal performance and proven results: High-density, high-purity graphite is the industry standard and often provides the most reliable outcome for general applications.
- If your primary concern is minimizing contamination of any kind: You must analyze the full impurity profile of both the crucible material and your raw alloy to ensure chemical compatibility.
Ultimately, your crucible selection directly defines the quality ceiling for your entire purification process.
Summary Table:
| Key Requirement | Critical Function | Ideal Material Examples |
|---|---|---|
| Extreme Chemical Inertness | Prevents reaction with molten Al-Mg alloy, avoiding contamination and crucible degradation. | High-purity graphite, high-purity alumina (corundum) |
| High-Temperature Stability | Maintains structural integrity at ~1373 K (1100°C) under high vacuum (10-15 Pa). | High-density graphite, advanced ceramics |
| High Purity & Non-Contamination | Ensures final metal purity by leaching no impurities into the melt. | Ultra-pure graphite, high-grade ceramics |
Achieve Unmatched Purity in Your Aluminum-Magnesium Alloy Refining
Your vacuum distillation process is only as reliable as your crucible. Contamination or crucible failure can compromise your entire batch and impact product quality. At KINTEK, we understand the extreme demands of high-temperature, high-vacuum metal processing.
Why Choose KINTEK Crucibles?
- Expertly Engineered Materials: Our crucibles are crafted from high-purity graphite and advanced ceramics, specifically selected for exceptional chemical inertness against molten aluminum and magnesium.
- Built for Extreme Conditions: They deliver superior thermal stability and structural integrity at temperatures up to 1373 K (1100°C) under high vacuum, ensuring consistent performance batch after batch.
- Guaranteed Purity: We prioritize material purity to prevent contamination, helping you achieve the high-quality separation of aluminum and magnesium you require.
Ready to optimize your process with a crucible designed for success? Contact our experts today for a personalized consultation. Let us help you select the perfect crucible material for your specific alloy and purity goals.
Visual Guide
Related Products
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the advantages of individually temperature-controlled zones in multi-zone furnaces? Unlock Precision Thermal Gradients
- How are multi zone tube furnaces applied in biomedical research? Unlock Advanced Biomaterial Engineering
- What safety precautions should be followed when operating a multi zone tube furnace? Ensure Safe and Efficient Lab Operations
- How do multi zone tube furnaces improve laboratory efficiency? Boost Throughput with Parallel Processing
- How are multi zone tube furnaces used in ceramics, metallurgy and glass research? Unlock Precise Thermal Control for Advanced Materials



















