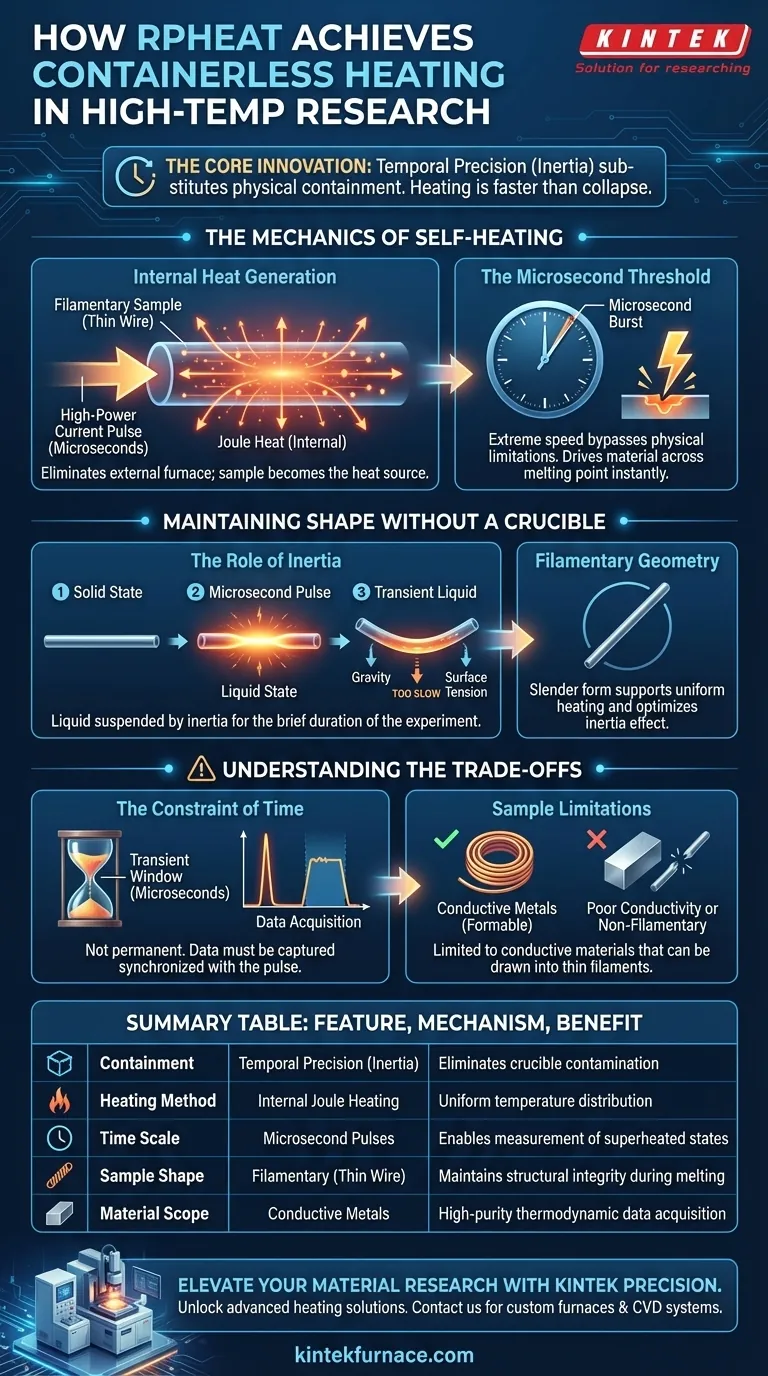
Rapid Resistance Pulse Heating (RPHeat) achieves containerless conditions by utilizing extreme speed and the physical principle of inertia rather than external supports or levitation. By injecting a high-power current pulse lasting only microseconds into a thin wire sample, the material generates its own internal heat via resistance. This process occurs so rapidly that the sample melts but temporarily retains its original shape, allowing for measurements before gravity or surface tension can deform it.
The core innovation of RPHeat is substituting physical containment with temporal precision. By heating a sample faster than it can physically collapse, researchers can measure the properties of liquid metals in a superheated state without the contamination risks associated with crucibles.

The Mechanics of Self-Heating
Internal Heat Generation
RPHeat eliminates the need for an external furnace by making the sample the heat source itself.
A powerful electric current is injected directly into a filamentary (thin wire) sample.
The sample’s natural electrical resistance converts this current into Joule heat, raising the temperature uniformly from within.
The Microsecond Threshold
The system delivers energy in extremely short bursts, typically measuring in microseconds.
This rapid energy injection drives the material across its melting point almost instantly.
The speed of this transition is critical to bypassing the physical limitations that usually require a container.
Maintaining Shape Without a Crucible
The Role of Inertia
The primary mechanism for "containerless" operation in RPHeat is physical inertia.
Although the sample transitions to a liquid state, the heating pulse is faster than the time required for the liquid to flow or change shape.
Essentially, the liquid metal remains suspended in its original filamentary form for the brief duration of the experiment.
Filamentary Geometry
The technique relies on the sample being a slender filament.
This specific geometry supports the uniform distribution of current and heat during the pulse.
It also optimizes the inertia effect, ensuring the sample holds its structural integrity long enough for data capture.
Understanding the Trade-offs
The Constraint of Time
Unlike magnetic or electrostatic levitation, RPHeat does not offer a permanent containerless state.
The "containerless" window is transient, lasting only as long as the inertial forces outweigh gravity and surface tension.
Data acquisition systems must be synchronized perfectly with the microsecond pulse to capture valid measurements.
Sample Limitations
This method is strictly limited to conductive materials capable of being formed into thin filaments.
Materials with poor conductivity or those that cannot be drawn into a wire may not generate sufficient Joule heat or maintain the necessary shape.
Making the Right Choice for Your Research
If you are investigating the properties of metals at high temperatures, RPHeat offers distinct advantages depending on your specific data requirements.
- If your primary focus is Purity: This method is ideal because the absence of a physical crucible prevents chemical reactions or contamination between the sample and a container.
- If your primary focus is Thermodynamics: The ability to reach superheated states allows for the precise measurement of volume changes and electrical properties that are impossible to capture in slow-heating scenarios.
By exploiting the lag between melting and physical deformation, RPHeat allows you to access a pristine, albeit fleeting, window into the physics of liquid metals.
Summary Table:
| Feature | RPHeat Mechanism | Benefit for Researchers |
|---|---|---|
| Containment | Temporal Precision (Inertia) | Eliminates crucible contamination |
| Heating Method | Internal Joule Heating | Uniform temperature distribution |
| Time Scale | Microsecond Pulses | Enables measurement of superheated states |
| Sample Shape | Filamentary (Thin Wire) | Maintains structural integrity during melting |
| Material Scope | Conductive Metals | High-purity thermodynamic data acquisition |
Elevate Your Material Research with KINTEK Precision
Unlock the full potential of high-temperature physics with advanced heating solutions tailored to your laboratory's specific requirements. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized high-temp furnaces—all fully customizable to support your unique experimental setups.
Whether you are studying the thermodynamic properties of liquid metals or developing new conductive materials, our equipment provides the stability and control necessary for groundbreaking results. Contact us today to discuss your research goals and discover how our custom-engineered furnace solutions can bring unmatched purity and precision to your lab.
Visual Guide
References
- Eleftheria Ntonti, Manabu Watanabe. Reference Correlations for the Density and Thermal Conductivity, and Review of the Viscosity Measurements, of Liquid Titanium, Zirconium, Hafnium, Vanadium, Niobium, Tantalum, Chromium, Molybdenum, and Tungsten. DOI: 10.1007/s10765-023-03305-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is vacuum degassing necessary for ZIF-8 impregnation? Achieve Uniform Macroporous Material Synthesis
- What is the role of high-vacuum sealing technology? Precision Synthesis of High-Performance Copper Sulfide
- What is the operational mechanism of a smelting reduction furnace (SRF)? Optimize Your HAlMan Metallurgy Process
- What is the significance of using different sizes of steel working ampoules? Precision vs. Efficiency in Lab Research
- What types of materials can crucible furnaces melt? Unlock the Power of Versatile Melting
- What is the necessity of in-situ DRIFTS in formaldehyde oxidation? Uncover Real-Time Catalytic Reaction Mechanisms
- What is the purpose of using a laboratory oven during sugarcane bagasse ash preparation? Optimize Material Pretreatment
- What is the primary function of an industrial drying oven for GBC? Achieving Material Standardization and Quality



















