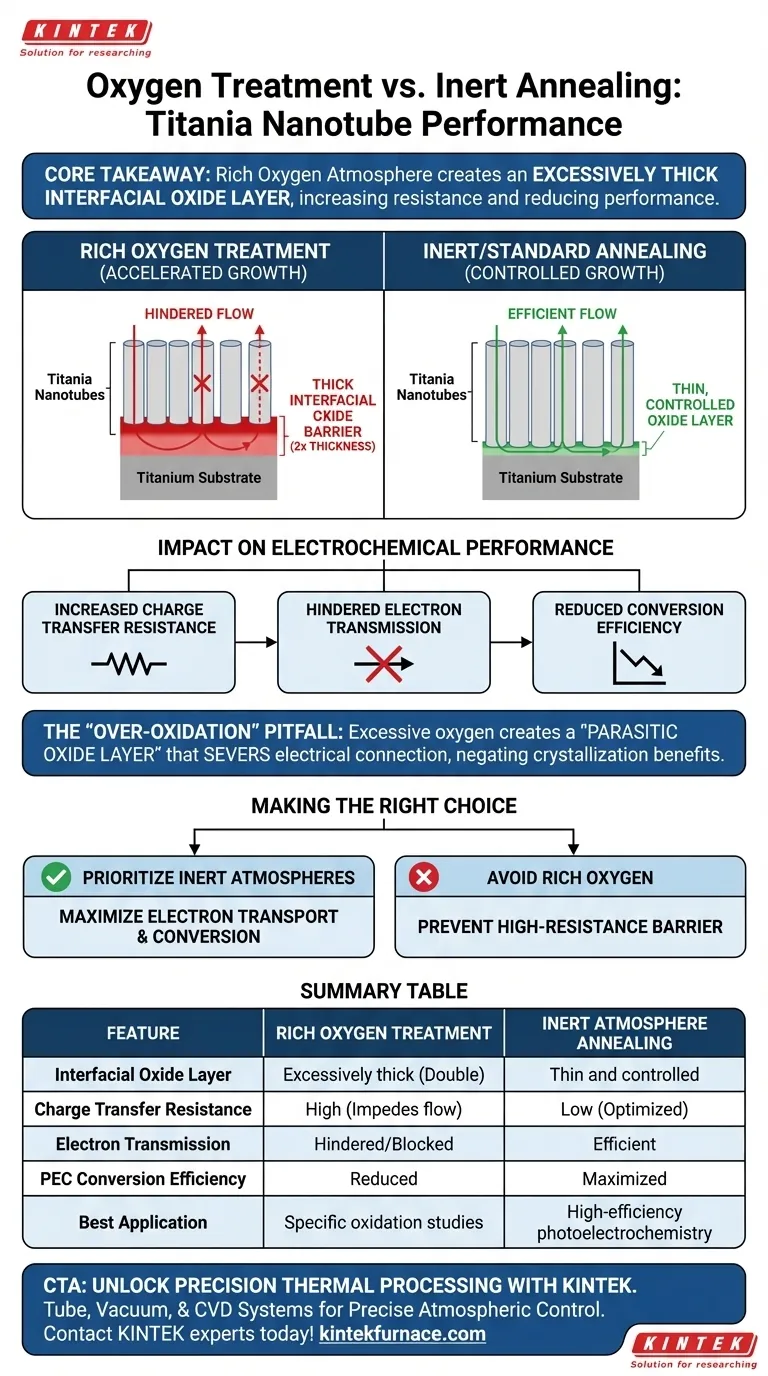
Treating titania nanotubes in a rich oxygen atmosphere fundamentally alters the structural interface between the nanotubes and the titanium substrate. Compared to annealing in inert or less aggressive atmospheres, using a rich oxygen environment in a tube furnace accelerates the thermal oxidation process, creating a significantly thicker barrier at the nanotube base.
Core Takeaway While oxidation is necessary for crystallization, a rich oxygen environment creates an excessively thick interfacial oxide layer (typically double the thickness of inert annealing). This thick barrier increases charge transfer resistance and blocks electron flow, directly reducing photoelectrochemical conversion performance.

The Mechanism of Structural Change
Accelerated Interfacial Growth
When you heat treat titania nanotubes in a rich oxygen environment, the abundance of oxygen accelerates the reaction rates at the metal-oxide interface.
This process specifically targets the boundary where the nanotubes meet the underlying titanium foil.
Doubling the Oxide Barrier
The primary physical outcome of this treatment is the thickening of the thermal oxidation layer.
According to technical benchmarks, this layer grows to be approximately twice as thick as the layer produced under inert atmospheres.
Impact on Electrochemical Performance
Increased Charge Transfer Resistance
The thickened oxide layer acts as an electrical resistor within your material stack.
Because the layer is excessively thick, it impedes the movement of charge carriers, significantly increasing the total charge transfer resistance of the system.
Hindered Electron Transmission
For photoelectrochemical applications, efficient electron transport from the nanotube to the substrate is critical.
The thick interfacial layer created by oxygen treatment acts as a physical blockage, hindering the transmission of electrons to the titanium substrate.
Reduced Conversion Efficiency
The cumulative effect of high resistance and blocked electron flow is a measurable drop in performance.
Consequently, samples treated in rich oxygen environments exhibit reduced photoelectrochemical conversion capabilities compared to those with thinner interfacial layers.
Understanding the Trade-offs
The "Over-Oxidation" Pitfall
It is a common misconception that more oxygen always leads to better stoichiometry or crystallinity during annealing.
While oxygen is required to convert amorphous titania to anatase or rutile phases, an excessive partial pressure of oxygen during the heat ramp creates a parasitic oxide layer.
This layer negates the benefits of crystallization by chemically severing the electrical connection between your active material (the nanotubes) and your current collector (the substrate).
Making the Right Choice for Your Goal
To optimize your titania nanotube fabrication, consider the following based on your specific performance metrics:
- If your primary focus is maximizing electron transport: Avoid rich oxygen environments to prevent the formation of a high-resistance interfacial barrier.
- If your primary focus is photoelectrochemical conversion: Prioritize annealing atmospheres that limit interfacial oxidation (such as inert gases) to maintain a thin, conductive junction between the tube and the substrate.
Control the atmosphere to balance crystallization with interfacial connectivity for optimal results.
Summary Table:
| Feature | Rich Oxygen Treatment | Inert Atmosphere Annealing |
|---|---|---|
| Interfacial Oxide Layer | Excessively thick (Double) | Thin and controlled |
| Charge Transfer Resistance | High (Impedes flow) | Low (Optimized) |
| Electron Transmission | Hindered/Blocked | Efficient |
| PEC Conversion Efficiency | Reduced | Maximized |
| Best Application | Specific oxidation studies | High-efficiency photoelectrochemistry |
Unlock Precision Thermal Processing with KINTEK
Don't let over-oxidation compromise your material research. KINTEK provides industry-leading Tube, Vacuum, and CVD furnace systems engineered for precise atmospheric control. Our expert-backed R&D and manufacturing capabilities ensure your titania nanotubes achieve the perfect balance of crystallinity and conductivity. Whether you need standard lab equipment or a fully customizable high-temperature solution, our systems are designed to meet the unique needs of advanced material scientists.
Ready to optimize your annealing environment? Contact KINTEK experts today to find the perfect furnace for your lab!
Visual Guide
References
- Younggon Son, Kiyoung Lee. Interfacial Charge Transfer Modulation via Phase Junctions and Defect Control in Spaced TiO <sub>2</sub> Nanotubes for Enhanced Photoelectrochemical Water Splitting. DOI: 10.1002/solr.202500334
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the technical significance of a horizontal tube furnace with a sliding rail for NiOx annealing? Enhance Control
- Why is an industrial monitoring camera necessary for measuring aluminum powder ignition delay in a tube furnace?
- How are tube furnaces designed for temperatures exceeding 1200°C? Unlock High-Temp Precision with Advanced Elements
- What are the key challenges in using tubular furnaces for materials science? Overcome Temperature, Uniformity, and Contamination Issues
- What temperature control features do tube turnouts typically have? Achieve Precise Thermal Management for Your Lab
- How does the industrial tube furnace contribute to Fe-N-C catalyst synthesis? Master High-Temperature Carbonization
- In which industries is the tube furnace commonly used? Essential for Materials Science, Energy, and More
- Can an alumina tube furnace be used for controlled atmosphere experiments? Yes, for precise high-temperature control.



















