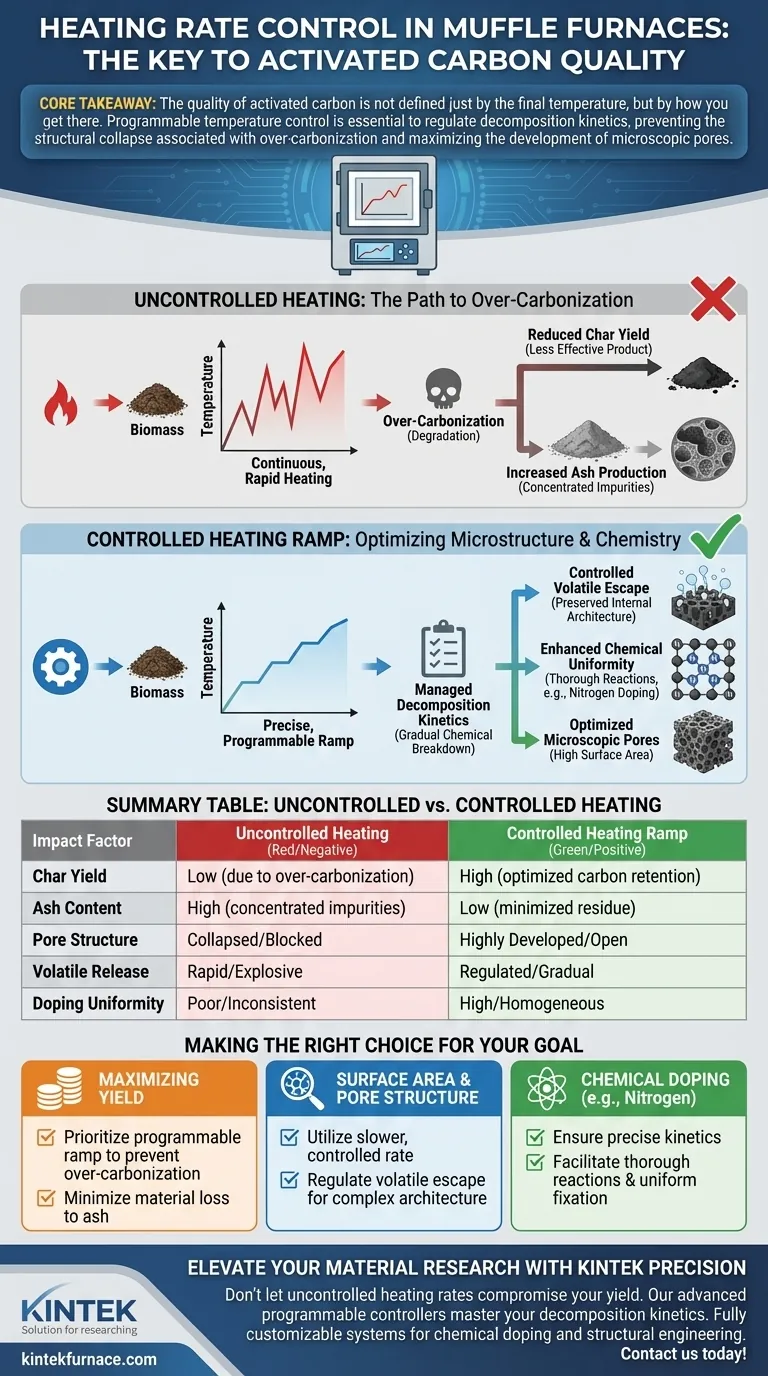
The control of the heating ramp within a muffle furnace is the single most critical variable for determining the yield and structural integrity of activated carbon. Without precise, programmable control, continuous heating accelerates the process too aggressively, resulting in "over-carbonization" of the biomass. This error directly leads to increased ash production and a significant reduction in the yield of effective char.
Core Takeaway: The quality of activated carbon is not defined just by the final temperature, but by how you get there. Programmable temperature control is essential to regulate decomposition kinetics, preventing the structural collapse associated with over-carbonization and maximizing the development of microscopic pores.

The Consequences of Uncontrolled Heating
The Mechanism of Over-Carbonization
When biomass is subjected to continuous, unregulated heating, the material often degrades past the point of optimal carbonization. This phenomenon, known as over-carbonization, burns off valuable carbon material that should have formed the structural backbone of the product.
Reduction in Char Yield
The immediate physical result of an uncontrolled heating rate is a drop in usable output. As the biomass over-carbonizes, the ratio of effective char decreases, leaving you with less product for the same amount of raw input.
Increased Ash Production
Alongside reduced yield, rapid or uncontrolled heating concentrates inorganic residues. This leads to a higher percentage of ash in the final product, which is an impurity that can block pores and degrade the adsorptive performance of the carbon.
Optimizing Microstructure and Chemistry
Managing Decomposition Kinetics
High-performance activated carbon requires the careful management of decomposition kinetics. A controlled, slower heating rate ensures that the chemical breakdown of biomass components happens gradually rather than chaotically.
Controlling Volatile Escape
Precisely regulated heating allows for the controlled release of volatile matter. By preventing the rapid, explosive escape of gases, you preserve the internal architecture of the material, optimizing the microscopic pore structure necessary for high surface area.
Enhancing Chemical Uniformity
Beyond physical structure, the heating rate dictates chemical composition. Controlled thermal processing facilitates more thorough reactions between precursors, which enhances the uniformity of elements such as nitrogen (doping) within the carbon matrix.
Understanding the Trade-offs
Equipment Capability vs. Cost
Achieving this level of quality requires specific equipment capabilities. Standard furnaces without programmable ramping features are often insufficient; an experimental furnace with precise, programmable temperature control is decisive for achieving consistent results.
Process Time vs. Quality
Implementing a slower, controlled ramp prolongs the overall processing time. While this increases the duration of the manufacturing cycle, it is a necessary investment to ensure thorough reactions and prevent the structural defects caused by rapid thermal shock.
Making the Right Choice for Your Goal
To maximize the value of your activated carbon production, align your heating strategy with your specific performance metrics.
- If your primary focus is Maximizing Yield: Prioritize a programmable ramp that specifically targets the prevention of over-carbonization to minimize material loss to ash.
- If your primary focus is Surface Area and Pore Structure: Utilize a slower, controlled heating rate to regulate the escape of volatiles and allow for the development of a complex microscopic architecture.
- If your primary focus is Chemical Doping (e.g., Nitrogen): Ensure your furnace can maintain precise kinetics to facilitate thorough reactions and uniform fixation of doping atoms.
Precise thermal regulation transforms the muffle furnace from a simple heating element into a tool for molecular engineering.
Summary Table:
| Impact Factor | Uncontrolled Heating | Controlled Heating Ramp |
|---|---|---|
| Char Yield | Low (due to over-carbonization) | High (optimized carbon retention) |
| Ash Content | High (concentrated impurities) | Low (minimized residue) |
| Pore Structure | Collapsed/Blocked | Highly Developed/Open |
| Volatile Release | Rapid/Explosive | Regulated/Gradual |
| Doping Uniformity | Poor/Inconsistent | High/Homogeneous |
Elevate Your Material Research with KINTEK Precision
Don’t let uncontrolled heating rates compromise your activated carbon yield. KINTEK provides industry-leading muffle, tube, and vacuum furnaces equipped with advanced programmable controllers to master your decomposition kinetics.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet the rigorous demands of chemical doping and structural engineering. Contact us today to discover how our high-temperature solutions can bring precision to your laboratory and maximize your production efficiency.
Visual Guide
References
- Charlotte Santana Velame, Ary Rocha Bezerra. Application of Activated Carbon Produced from Licuri Bark (Syagrus coronata) in Water Filtration. DOI: 10.34178/jbth.v8i2.476
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What role does an industrial microwave muffle furnace play in the sintering process of porous mullite ceramic skeletons?
- How are muffle furnaces used in the pharmaceutical industry? Essential for Quality Control and R&D
- What process function does a high-temperature muffle furnace perform in pre-sintering spinel ceramics?
- Why is a high-temperature muffle furnace required for the secondary calcination of SC-NMNO? Key to Single Crystals
- What roles do the electric muffle furnace and gas-tight retort play in biochar production? Master Controlled Pyrolysis
- What is the role of a high-temperature muffle furnace in Mg-Zn-Al LDH transformation? Unlocking Adsorption Power
- How does an industrial-grade muffle furnace contribute to the catalyst activation process? Maximize Catalyst Efficiency
- How is a muffle furnace utilized in the preparation of carbon materials derived from L-valine? Master Carbonization



















