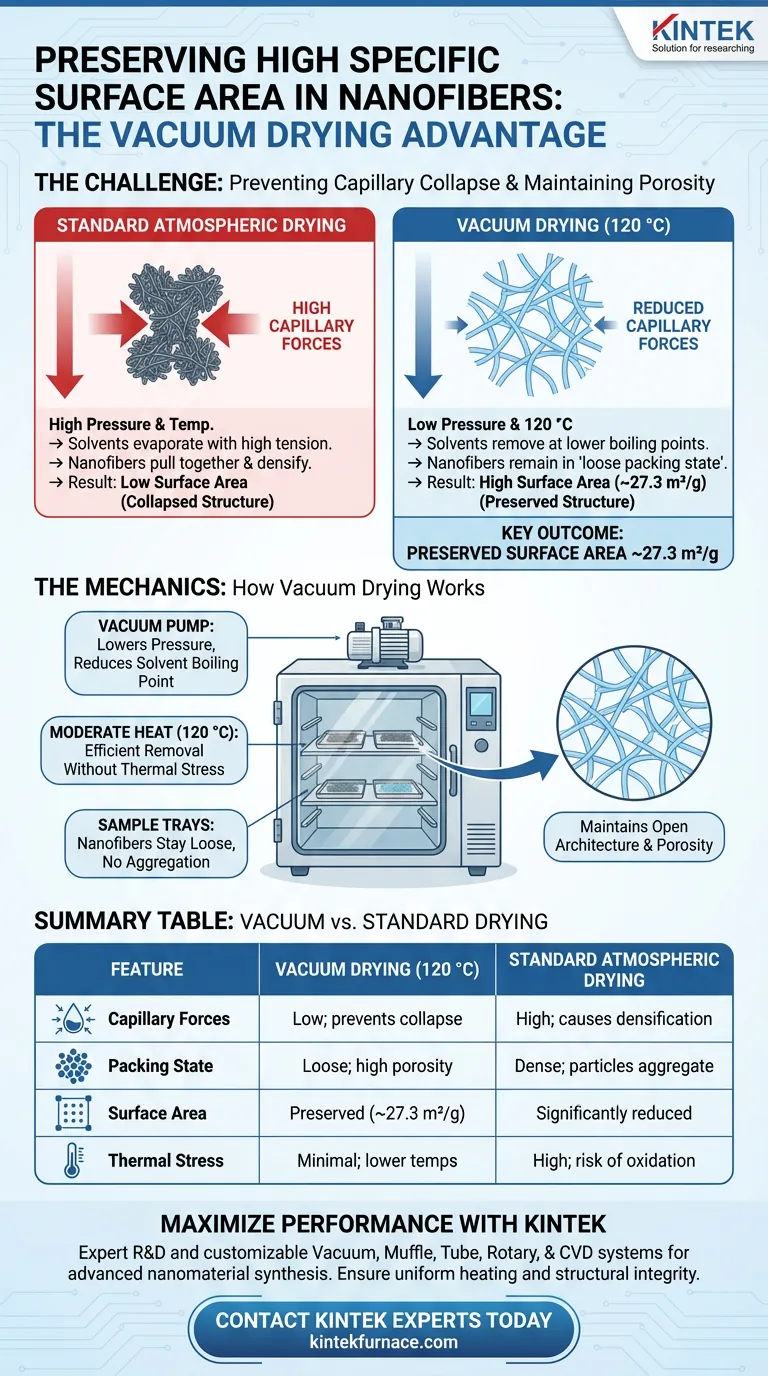
Vacuum drying preserves the structural integrity of nanofibers by facilitating the removal of solvents at reduced pressures. specifically for synthesized (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 nanofibers, this method allows for drying at 120 °C, which eliminates adsorbed moisture and residual solvents without forcing the particles to aggregate. This process is critical for preventing capillary collapse, thereby maintaining the material's high specific surface area.
By lowering the pressure, the vacuum drying oven reduces the boiling point of solvents, preventing the severe capillary forces associated with standard evaporation. This ensures the nanofibers remain in a "loose packing state" rather than collapsing into a dense mass, preserving a specific surface area of approximately 27.3 m²/g.

The Mechanics of Surface Area Preservation
Preventing Capillary Collapse
When solvents evaporate from a nanomaterial under normal atmospheric pressure, the receding liquid creates high surface tension. This tension generates strong capillary forces that pull the delicate nanostructures together.
A vacuum drying oven mitigates this by removing solvents at low pressure. This significantly reduces the capillary forces exerted on the pore walls, preventing the structure from collapsing inward and preserving the material's porosity.
Maintaining a Loose Packing State
For high-performance applications, nanofibers must not clump together. The vacuum drying process ensures that the precipitate does not densify during the drying phase.
By avoiding densification, the nanofibers maintain a loose packing state. This open architecture is directly responsible for achieving and sustaining the high specific surface area of 27.3 m²/g found in (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3.
Efficient Removal at Lower Temperatures
Vacuum drying allows for the thorough removal of stubborn solvents and adsorbed moisture at a moderate temperature of 120 °C.
Because the vacuum lowers the boiling point of liquids, the material does not need to be subjected to excessive heat to achieve complete dryness. This protects the chemical stability of the fibers while ensuring the surface is free of contaminants that could block active sites.
Understanding the Trade-offs
The Risk of Standard Drying
It is important to understand why standard thermal drying is often unsuitable for this application. Drying without a vacuum would require higher temperatures to remove the same amount of solvent.
Thermal Sensitivity and Oxidation
While (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 is relatively robust, relying on high heat to drive off solvents increases the risk of oxidation or unwanted phase transformations. Vacuum drying minimizes this thermal stress, preserving the material's intended phase and morphology.
Making the Right Choice for Your Synthesis
To ensure you achieve the target material properties, align your drying method with your specific goals:
- If your primary focus is Surface Area: Use vacuum drying to prevent capillary collapse and lock in the ~27.3 m²/g specific surface area required for high reactivity.
- If your primary focus is Purity: Rely on the vacuum environment to completely strip residual solvents and moisture at 120 °C without resorting to potentially damaging high temperatures.
Vacuum drying is not merely a dehydration step; it is a structural preservation technique essential for maintaining the performance potential of your nanofibers.
Summary Table:
| Feature | Vacuum Drying (120 °C) | Standard Atmospheric Drying |
|---|---|---|
| Capillary Forces | Low; prevents structure collapse | High; causes densification |
| Packing State | Loose; maintains high porosity | Dense; particles aggregate |
| Surface Area | Preserved (approx. 27.3 m²/g) | Significantly reduced |
| Thermal Stress | Minimal; lower boiling points | High; risk of oxidation/phase change |
Maximize Your Material Performance with KINTEK
Preserving the delicate architecture of (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 nanofibers requires more than just heat—it requires precision environmental control. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Vacuum, Muffle, Tube, Rotary, and CVD systems, all of which are fully customizable to meet the rigorous demands of advanced nanomaterial synthesis.
Whether you are scaling up production or refining laboratory results, our high-temp furnaces ensure uniform heating and structural integrity for your most sensitive samples. Don’t let capillary collapse compromise your research.
Contact KINTEK Experts Today to find the ideal drying and heating solution for your unique needs.
Visual Guide
References
- Paweł A. Krawczyk, Władysław W. Kubiak. Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation. DOI: 10.3390/ma17081883
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the process of vacuum annealing? Achieve Superior Material Purity and Performance
- Why is a vacuum heating and cooling stage necessary for SWCNT research? Unlock Precision in Thermal Conductivity
- How do vacuum chambers and heating systems prepare zirconium alloy samples? Achieving Precise Hydrogen Concentration
- What is the purpose of using industrial vacuum furnaces for 3003mod aluminum? Optimize H14 Temper & Material Stress
- What is the function of a high-vacuum furnace in tantalum carburization? Purity & Reaction Precision
- What materials are suitable for sintering in a vacuum furnace? Unlock High Purity and Strength
- What improvements do sintering furnaces with VGF functionality offer? Elevate Crystal Purity and Structural Integrity
- What are the benefits of vacuum heat treatment? Achieve Superior Metallurgical Control



















