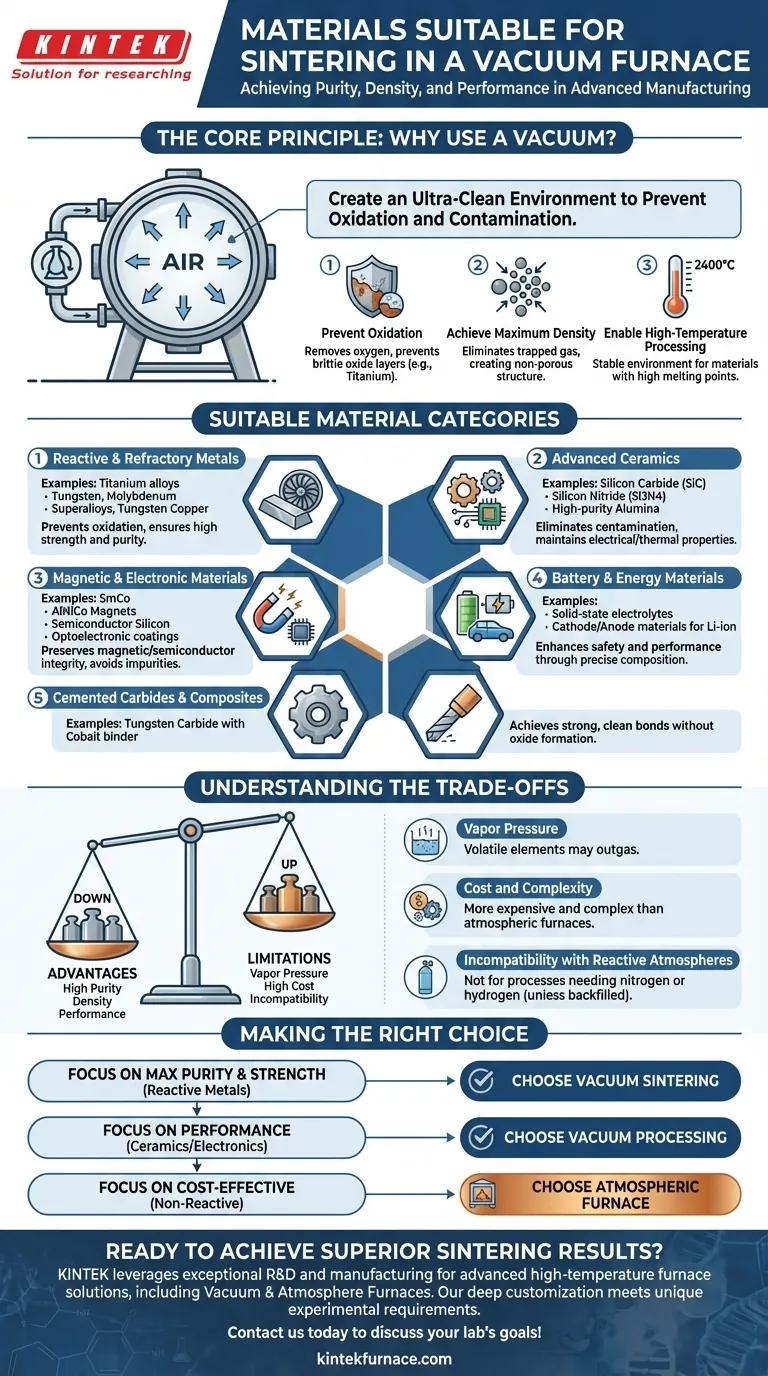
In short, vacuum furnaces are used to sinter materials that are highly reactive with atmospheric gases like oxygen and nitrogen, especially at high temperatures. This includes a broad range of advanced metals, ceramics, and electronic materials where purity and density are critical for performance.
The core purpose of vacuum sintering is not just to heat a material, but to create an ultra-clean environment that prevents oxidation and contamination. This allows powdered materials to fuse together at extreme temperatures, achieving a final density and strength that would be impossible in a normal atmosphere.

The Core Principle: Why Use a Vacuum?
To understand which materials are suitable, you must first understand the problem a vacuum furnace solves. At the high temperatures required for sintering, many materials react aggressively with the air around them. A vacuum removes that air.
Preventing Oxidation and Contamination
Many high-performance materials, like titanium alloys or superalloys, are prone to oxidation when heated. This forms a brittle oxide layer that severely degrades the material's structural integrity and performance.
A vacuum environment removes the oxygen and other reactive gases, ensuring the material remains pure throughout the heating and bonding process.
Achieving Maximum Density and Purity
Sintering works by bonding powder particles together. If air is present, gases can become trapped between particles, creating voids (porosity) in the final product and weakening it.
By removing the air, a vacuum allows for superior densification. This is critical for components that require maximum strength and a non-porous structure.
Enabling High-Temperature Processing
Vacuum furnaces are engineered with advanced heating elements capable of reaching temperatures up to 2400°C.
Many refractory metals and advanced ceramics have extremely high melting points. A vacuum is one of the few environments that can remain stable and non-reactive at the temperatures needed to process them.
A Breakdown of Suitable Material Categories
Based on these principles, vacuum sintering is the ideal method for several distinct classes of materials.
Reactive and Refractory Metals
This category includes materials with high melting points or a strong affinity for oxygen.
Examples include titanium alloys, tungsten, molybdenum, superalloys, and tungsten copper alloys. A vacuum is non-negotiable for achieving their desired mechanical properties.
Advanced Ceramics
High-purity technical ceramics require a controlled environment to prevent contamination that would compromise their unique electrical or thermal properties.
Suitable materials are silicon carbide (SiC), silicon nitride (Si3N4), and high-purity alumina (Al2O3).
Magnetic and Electronic Materials
The performance of these materials is extremely sensitive to impurities. Even trace amounts of oxygen can ruin their magnetic or semiconductor properties.
This group includes samarium cobalt (SmCo), aluminum nickel cobalt (AlNiCo) magnets, semiconductor materials like silicon, and various optoelectronic coating materials.
Battery and Energy Materials
Modern energy storage relies on materials with precise chemical compositions.
Vacuum sintering is used for next-generation solid-state electrolytes as well as cathode and anode materials for lithium-ion batteries, where purity is directly linked to performance and safety.
Cemented Carbides and Composites
Cemented carbides (hard materials used for cutting tools) and other metal-matrix composites are often sintered in a vacuum.
This ensures a strong, clean bond between the hard ceramic particles (like tungsten carbide) and the metal binder (like cobalt) without forming undesirable oxides at the interface.
Understanding the Trade-offs
While powerful, vacuum sintering is not a universal solution. The process has specific limitations that make it unsuitable for certain applications.
The Issue of Vapor Pressure
The primary limitation is outgassing. Under a vacuum, elements with a high vapor pressure can "boil off" from the material at high temperatures, altering the final chemical composition of the alloy.
Materials containing volatile elements like zinc, cadmium, or manganese may not be suitable for high-vacuum sintering unless process parameters are carefully controlled.
Cost and Complexity
Vacuum furnace systems, with their associated pumps, robust chambers, and sophisticated controls, are significantly more expensive and complex to operate than standard atmospheric furnaces.
This higher cost is only justified when the material's properties demand the level of purity and density that a vacuum provides.
Incompatibility with Reactive Atmospheres
Some materials require a specific gas to achieve their final state. For example, some sintering processes are intentionally performed in a nitrogen or hydrogen atmosphere. A vacuum furnace is, by definition, unsuitable for these applications unless it is backfilled with the desired gas, a process known as atmosphere sintering.
Making the Right Choice for Your Goal
Selecting the right process depends entirely on your material and performance requirements.
- If your primary focus is maximum purity and strength for reactive metals (e.g., titanium, superalloys): Vacuum sintering is the essential, industry-standard method to prevent catastrophic oxidation.
- If your primary focus is the performance of advanced ceramics or electronic materials: Vacuum processing is critical for eliminating contaminants that would degrade their thermal, electrical, or magnetic properties.
- If your primary focus is cost-effective sintering of non-reactive powders (e.g., some iron or steel parts): A simpler and less expensive atmospheric furnace is often a more practical choice if minor oxidation is acceptable.
Ultimately, the decision to use a vacuum furnace is driven by the material's inherent need for a pristine processing environment.
Summary Table:
| Material Category | Examples | Key Benefits |
|---|---|---|
| Reactive and Refractory Metals | Titanium alloys, Tungsten, Molybdenum, Superalloys | Prevents oxidation, ensures high strength and purity |
| Advanced Ceramics | Silicon carbide (SiC), Silicon nitride (Si3N4), Alumina (Al2O3) | Eliminates contamination, maintains electrical/thermal properties |
| Magnetic and Electronic Materials | Samarium cobalt (SmCo), AlNiCo magnets, Semiconductor silicon | Preserves magnetic/semiconductor integrity, avoids impurities |
| Battery and Energy Materials | Solid-state electrolytes, Cathode/anode materials for Li-ion batteries | Enhances safety and performance through precise composition |
| Cemented Carbides and Composites | Tungsten carbide with cobalt binder | Achieves strong, clean bonds without oxide formation |
Ready to achieve superior sintering results with tailored vacuum furnace solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, delivering enhanced purity, density, and performance for materials like reactive metals, ceramics, and electronic components. Contact us today to discuss how we can support your lab's goals!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why is a high vacuum essential for Ti-6Al-4V sintering? Protect Your Alloys from Embrittlement
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity
- What tasks does a high-temperature vacuum sintering furnace perform for PEM magnets? Achieve Peak Density
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness



















