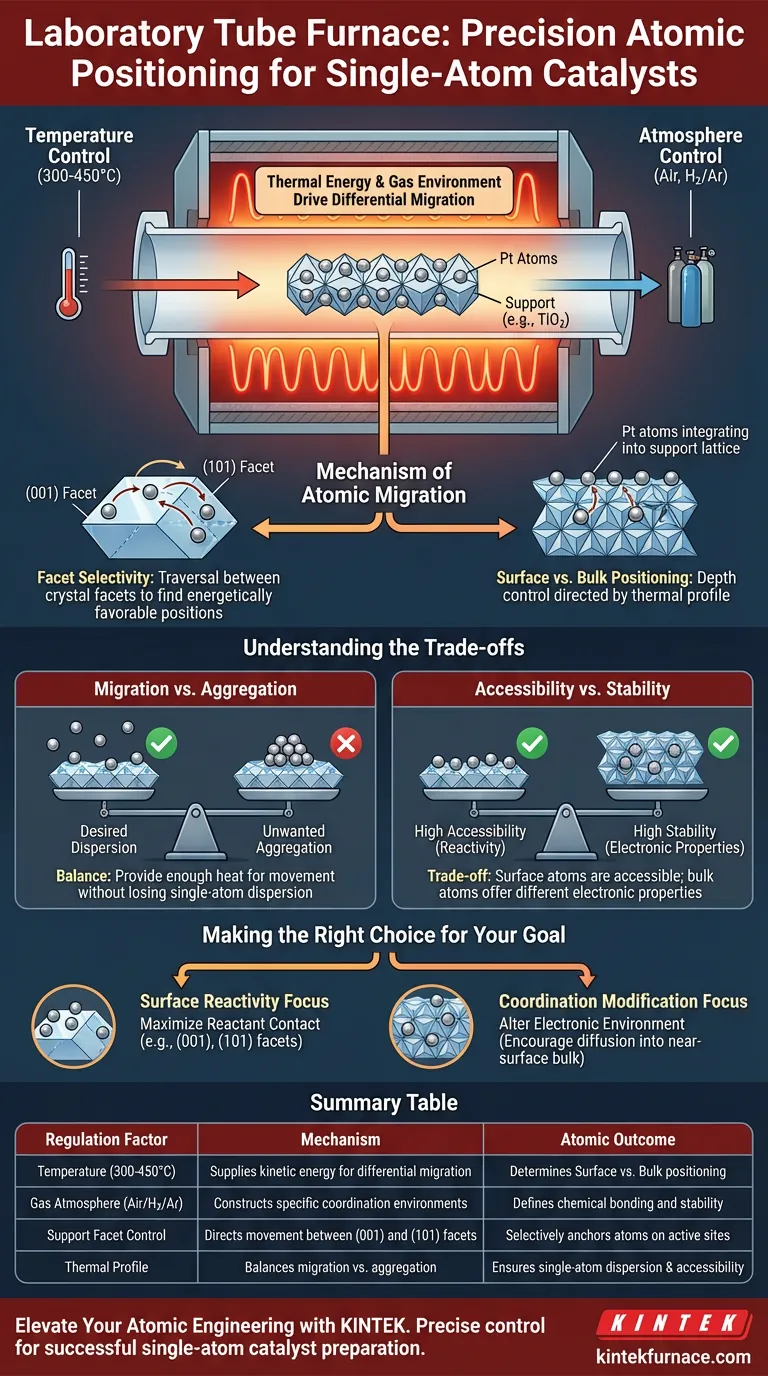
A laboratory tube furnace functions as a precision instrument for atomic-scale engineering, regulating the positioning of platinum (Pt) atoms through the rigorous control of temperature and atmosphere. By creating specific thermal profiles between 300°C and 450°C under flowing air or hydrogen/argon mixtures, the furnace provides the exact kinetic energy required to drive the differential migration of platinum atoms. This allows you to selectively anchor atoms on specific crystal facets or diffuse them into the support structure, effectively tailoring the catalyst's active sites.
The tube furnace does not merely heat the sample; it dictates the final atomic architecture of the catalyst. By manipulating thermal energy and gas environments, it forces platinum atoms to migrate to specific coordination environments, determining whether they reside on the surface or integrate into the bulk.

The Mechanism of Atomic Migration
Thermal Energy as a Driving Force
The primary role of the tube furnace is to supply controlled thermal energy, specifically during the calcination process.
Operating within a critical temperature window of 300°C to 450°C, the furnace imparts sufficient energy to mobilize the platinum atoms.
This energy drives differential migration, causing atoms to move across the support material rather than remaining static.
Facet Selectivity
The migration process is not random; it is influenced by the crystal structure of the support, such as titanium dioxide.
The thermal treatment enables platinum atoms to traverse different crystal facets, specifically moving between the (001) and (101) facets.
This movement allows the atoms to seek energetically favorable positions based on the temperature applied.
Surface vs. Bulk Positioning
The ultimate goal of this thermal regulation is to control the depth of the platinum atoms.
Depending on the specific heating profile, platinum atoms can be directed to reside strictly on the surface of the support.
Alternatively, the process can induce diffusion into the near-surface bulk, changing how the atom interacts with reactants.
The Role of Atmospheric Control
Gas Environment Influence
Temperature alone is insufficient; the chemical atmosphere within the tube is equally critical for positioning.
The furnace regulates this by maintaining a flow of specific gases, such as air or hydrogen/argon mixtures.
Defining Coordination Environments
The combination of gas flow and heat constructs distinct coordination environments for the platinum.
These environments define how the platinum is chemically bonded to the support, which is the fundamental factor in catalytic performance.
Understanding the Trade-offs
Migration vs. Aggregation
While thermal energy is necessary for migration, it presents a delicate balance.
The furnace must provide enough heat to move the atoms to the desired facets or bulk locations.
However, precise control is required to prevent unwanted changes in the support structure or loss of the desired single-atom dispersion.
Accessibility vs. Stability
There is an inherent trade-off in choosing where the platinum resides.
Positioning atoms on the surface generally maximizes their accessibility to reactants.
Conversely, diffusing atoms into the near-surface bulk may offer different electronic properties or stability, but potentially at the cost of immediate surface exposure.
Making the Right Choice for Your Goal
To optimize your single-atom catalyst preparation, you must align your furnace settings with your specific structural targets.
- If your primary focus is surface reactivity: Utilize thermal profiles that favor the stabilization of platinum atoms on the exposed crystal facets like (001) or (101) to maximize reactant contact.
- If your primary focus is coordination modification: Adjust the temperature and atmosphere to encourage diffusion into the near-surface bulk, altering the electronic environment of the platinum.
Mastering the correlation between thermal input and atomic migration allows you to transition from simple heating to true structural design.
Summary Table:
| Regulation Factor | Mechanism | Atomic Outcome |
|---|---|---|
| Temperature (300-450°C) | Supplies kinetic energy for differential migration | Determines Surface vs. Bulk positioning |
| Gas Atmosphere (Air/H2/Ar) | Constructs specific coordination environments | Defines chemical bonding and stability |
| Support Facet Control | Directs movement between (001) and (101) facets | Selectively anchors atoms on active sites |
| Thermal Profile | Balances migration vs. aggregation | Ensures single-atom dispersion & accessibility |
Elevate Your Atomic Engineering with KINTEK
Precise control over thermal profiles and gas environments is non-negotiable for successful single-atom catalyst preparation. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory needs.
Whether you are targeting surface reactivity or bulk diffusion, our advanced heating solutions provide the stability and precision required for your most sensitive materials. Contact us today to find the perfect furnace for your research!
Visual Guide
References
- Wenjie Zang, Xiaoqing Pan. Distribution of Pt single atom coordination environments on anatase TiO2 supports controls reactivity. DOI: 10.1038/s41467-024-45367-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does an alumina tube furnace work? Achieve Precise, Contamination-Free Heating
- What is the significance of using a vacuum tube furnace for Fe3Al powder? Optimize Magnetic Phase Transformation
- Why is an industrial tube furnace required for the heat treatment of SiCN(Ni)/BN ceramics? Master Precise Pyrolysis
- How does an Infrared Heating Rapid Scan Furnace facilitate accurate TDS measurements for hydrogen trapping?
- Why is it necessary to precisely control the oxygen flow rate in a tube furnace? Optimize Li-Deficient Composites
- What is the necessity of using sealed silica tubes in the BCM reduction method? Ensuring High-Purity Synthesis
- How does a specialized quartz heating furnace ensure accuracy? Mastering Thermoluminescence at High Temperatures
- How does the cooling rate of a high-temp tube furnace affect Cu-Zn disordered CZTS layers? Unlock Precise Cation Control



















