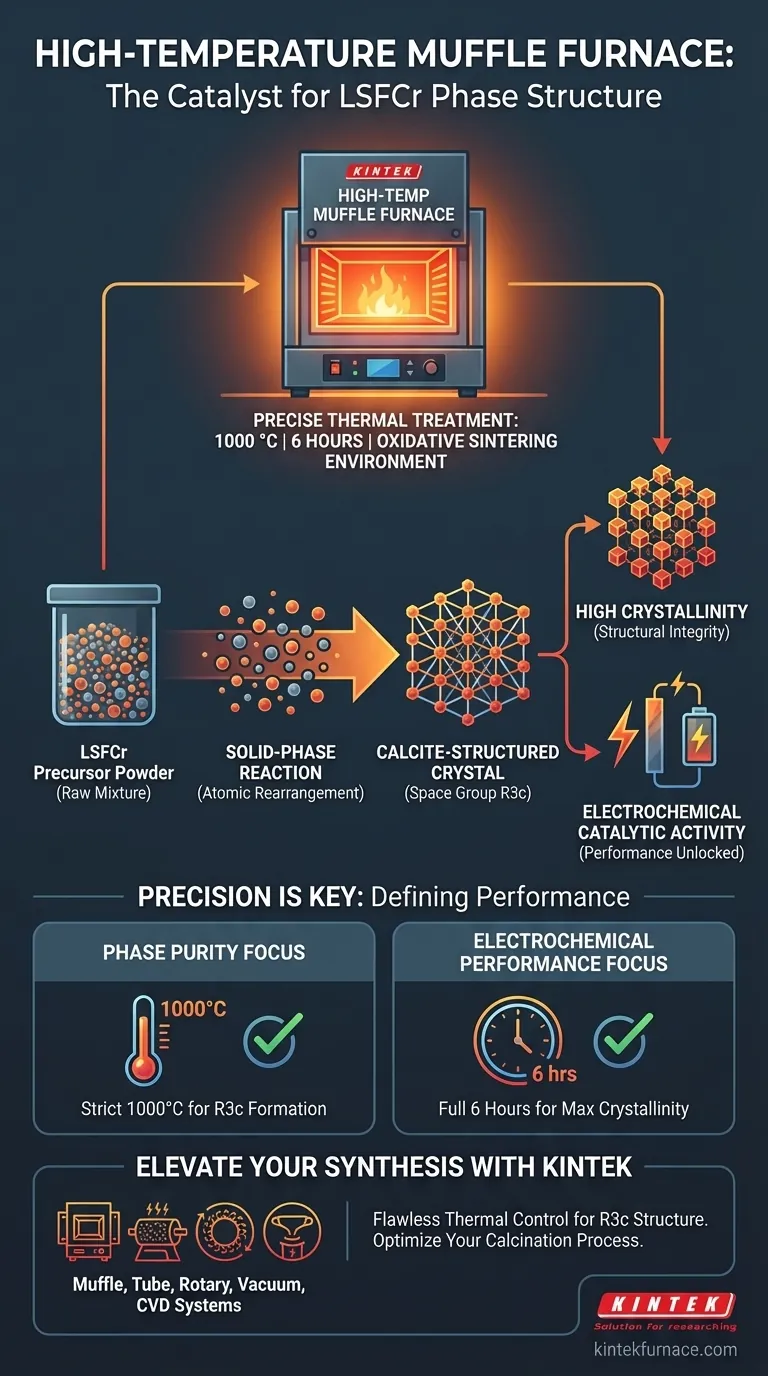
A high-temperature muffle furnace acts as the primary catalyst for phase transformation, providing a stable oxidative sintering environment at 1000 °C for a duration of 6 hours. This precise thermal treatment triggers a solid-phase reaction within the precursor material, which is the mechanism responsible for converting the raw powder into a specific calcite-structured crystal (space group R3c).
The furnace does not merely dry or harden the material; it orchestrates a chemical restructuring essential for high crystallinity. This controlled calcination is the decisive factor in unlocking the electrochemical catalytic activity required for effective LSFCr electrodes.

The Mechanism of Phase Formation
Triggering Solid-Phase Reactions
The muffle furnace creates an environment where thermal energy drives chemical changes without melting the material.
By maintaining a steady temperature of 1000 °C for 6 hours, the furnace supplies the energy required to initiate a solid-phase reaction.
This reaction rearranges the atomic structure of the precursor, moving it from a raw mixture to a unified crystalline lattice.
Achieving the Calcite Structure
The specific goal of this thermal process is the formation of a calcite-structured crystal.
Within the furnace, the material adopts a specific crystallographic symmetry known as the R3c space group.
This structural arrangement is not accidental; it is a direct result of the specific oxidative sintering conditions provided by the furnace.
Why This Process Defines Performance
The Role of Crystallinity
The quality of the electrode is defined by its crystallinity.
The muffle furnace ensures that the phase formation is complete and uniform, resulting in high crystallinity.
Without this precise thermal history, the material would lack the structural integrity required for its end use.
Unlocking Catalytic Activity
Structure dictates function in electrode materials.
The formation of the R3c phase is directly linked to the material's electrochemical catalytic activity.
Therefore, the calcination process in the muffle furnace is the "decisive step" that determines whether the final powder will perform effectively as an electrode.
Understanding the Constraints
The Necessity of Precision
While muffle furnaces are versatile, the LSFCr process relies on specific parameters.
The reference emphasizes a precise 6-hour duration at 1000 °C; deviating from this timeframe or temperature could result in incomplete phase formation.
Oxidative Environment Requirements
Unlike semiconductor annealing processes that may require inert atmospheres to prevent oxidation, this process requires oxidative sintering.
Operators must ensure the furnace allows for an oxygen-rich environment to facilitate the correct chemical changes in the LSFCr powder.
Making the Right Choice for Your Goal
To ensure you achieve the desired material properties, align your processing parameters with your specific objective:
- If your primary focus is Phase Purity: strictly adhere to the 1000 °C set point to guarantee the formation of the calcite-structured crystal (R3c).
- If your primary focus is Electrochemical Performance: ensure the dwell time reaches the full 6 hours to maximize crystallinity and catalytic activity.
By controlling the thermal variables of the muffle furnace, you directly control the functional quality of the final electrode material.
Summary Table:
| Parameter | Process Requirement | Impact on LSFCr Material |
|---|---|---|
| Temperature | 1000 °C | Initiates solid-phase reaction & atomic rearrangement |
| Duration | 6 Hours | Ensures complete phase transformation & high crystallinity |
| Atmosphere | Oxidative Sintering | Facilitates correct chemical restructuring |
| Space Group | R3c (Calcite) | Determines electrochemical catalytic activity |
| Outcome | Uniform Lattice | Provides structural integrity for electrode performance |
Elevate Your Electrode Material Synthesis with KINTEK
Precision is the decisive factor in unlocking the electrochemical potential of LSFCr powders. At KINTEK, we understand that achieving the R3c calcite structure requires flawless thermal control. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain stable oxidative environments up to 1000°C and beyond.
Whether you need standard lab equipment or a fully customizable high-temp furnace for unique material research, KINTEK provides the reliability your lab demands. Our systems ensure uniform heat distribution and precise dwell times, empowering you to maximize crystallinity and catalytic activity in every batch.
Ready to optimize your calcination process? Contact us today to find the perfect furnace solution for your lab!
Visual Guide
References
- Hao Dong, Zhaotong Wei. Study on Performance and Preparation of Lanthanum-Strontium-Iron-Chromium Electrodes for Using in Symmetric SOFC. DOI: 10.54097/8d6pg665
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the function of a laboratory high-temperature furnace in cook-off synthesis? A Precise Thermal Initiator
- What are some advancements in modern muffle furnace technology? Boost Precision and Efficiency in Your Lab
- Why is a laboratory muffle furnace core to CCT nanocatalyst prep? Optimize Your Calcination Results
- What is the role of a Muffle Furnace in the synthesis of PTI/LiCl? Achieve High-Crystallinity Poly(triazine imide)
- How does the temperature control system of a muffle furnace work? Achieve Precise Thermal Processing for Your Lab
- What is the primary function of a high-temperature muffle furnace for cerium dioxide precursors? Expert Calcination Tips
- What role do muffle furnaces play in semiconductor material processing? Essential for Precise Annealing and Dopant Activation
- How is a muffle furnace utilized in the drying treatment of BSCF catalyst precursors? Ensure Precision Phase Change



















