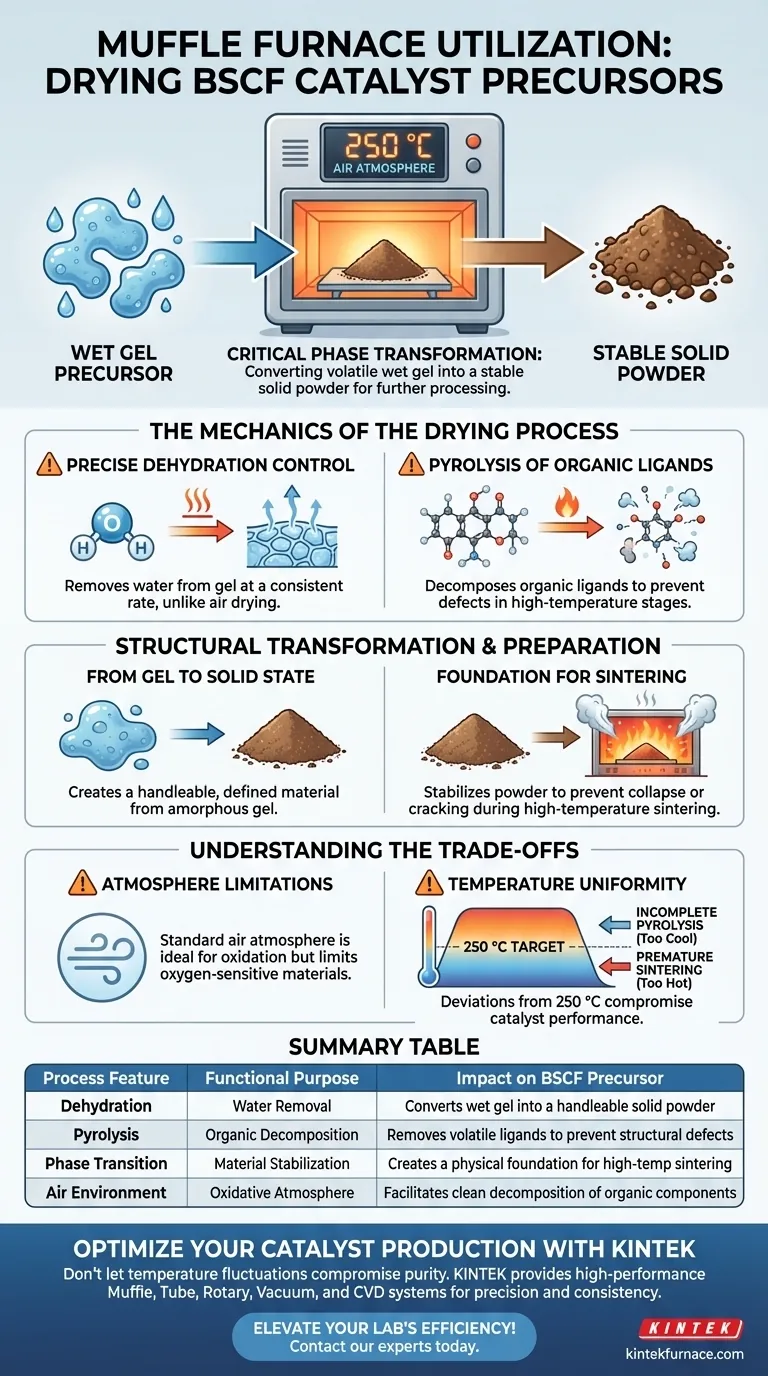
In the context of BSCF catalyst precursors, a muffle furnace is utilized to create a stable, high-temperature air environment, typically maintained at 250 °C. This specific thermal treatment is essential for dehydrating the initial wet gel and facilitating the pyrolysis of organic ligands, transforming the material into a solid powder suitable for further processing.
The muffle furnace serves as a critical phase-transformation tool, converting a volatile wet gel into a stable solid powder. By maintaining a precise 250 °C oxidative environment, it ensures the removal of moisture and organic components, establishing the physical foundation required for subsequent high-temperature sintering.

The Mechanics of the Drying Process
Precise Dehydration Control
The primary function of the muffle furnace in this application is controlled dehydration. Unlike simple air drying, the furnace provides a stable thermal field that removes water from the wet gel structure at a consistent rate.
Pyrolysis of Organic Ligands
Beyond removing water, the 250 °C environment triggers a chemical change known as pyrolysis. This process decomposes specific organic ligands present within the precursor matrix.
Removing these organics at this stage is crucial. It prevents uncontrolled combustion or structural defects that could occur if these volatile components were carried over into higher-temperature processing stages.
Structural Transformation and Preparation
From Gel to Solid State
The treatment effectively transitions the precursor from a gel state into a solid powder. This physical transformation creates a handleable, defined material from an amorphous or semi-solid starting point.
Foundation for Sintering
This drying step is not the final stage; it is the physical basis for sintering. By stabilizing the powder at 250 °C, the muffle furnace prepares the catalyst for the much higher temperatures required in subsequent sintering steps.
Without this intermediate stabilization, the rapid heating associated with sintering could cause the precursor structure to collapse or crack due to the rapid release of remaining steam or gases.
Understanding the Trade-offs
Atmosphere Limitations
A standard muffle furnace typically operates with an air atmosphere. While this is ideal for the oxidative decomposition required for BSCF precursors, it limits the ability to process materials that are sensitive to oxygen or require a reduction atmosphere during the drying phase.
Temperature Uniformity
While muffle furnaces are designed for stability, thermal gradients can exist within the chamber. If the temperature deviates significantly from the target 250 °C, you risk incomplete pyrolysis (if too cool) or premature sintering (if too hot), both of which compromise the final catalyst performance.
Making the Right Choice for Your Goal
To maximize the effectiveness of the drying treatment for BSCF precursors, consider the following:
- If your primary focus is material purity: Ensure the furnace provides sufficient airflow to evacuate the gases produced during the pyrolysis of organic ligands, preventing re-deposition on the powder.
- If your primary focus is structural homogeneity: Verify that the furnace maintains a strict temperature profile around 250 °C to ensure the wet gel dehydrates uniformly throughout the entire batch.
The muffle furnace acts not just as a heater, but as a stabilizing environment that defines the physical integrity of your final catalyst product.
Summary Table:
| Process Feature | Functional Purpose | Impact on BSCF Precursor |
|---|---|---|
| Dehydration | Water Removal | Converts wet gel into a handleable solid powder |
| Pyrolysis | Organic Decomposition | Removes volatile ligands to prevent structural defects |
| Phase Transition | Material Stabilization | Creates a physical foundation for high-temp sintering |
| Air Environment | Oxidative Atmosphere | Facilitates clean decomposition of organic components |
Optimize Your Catalyst Production with KINTEK
Don’t let temperature fluctuations compromise your material purity. At KINTEK, we understand that the drying and pyrolysis of BSCF precursors require absolute thermal stability. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for precision. Whether you need a standard lab furnace or a fully customizable system tailored to your unique high-temperature research, KINTEK delivers the reliability you need for consistent structural homogeneity.
Ready to elevate your lab's efficiency? Contact our experts today to find the perfect thermal solution!
Visual Guide
References
- Weijie Cao, Yoshiharu Uchimoto. Elucidation of the factors governing the oxygen evolution reaction in Ba<sub>0.5</sub>Sr<sub>0.5</sub>Co<sub><i>x</i></sub>Fe<sub>1−<i>x</i></sub>O<sub>3−<i>δ</i></sub> catalysts <i>via operando</i> hard and soft X-ray absorption spectroscopy. DOI: 10.1039/d5cy00056d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a high-temperature box resistance furnace play in converting LDHs into LDOs? Essential Thermal Insights
- How are muffle furnaces utilized in high-temperature sintering within the pharmaceutical industry? Unlock Precision in Drug Delivery and Implants
- What makes a digital muffle furnace indispensable for high-temperature applications? Discover Precision and Purity for Your Lab
- How does the insulation in a muffle furnace contribute to its efficiency? Unlock Energy Savings and Precision
- What is the technical necessity of using high-temperature furnaces for neutron scattering? Ensure Sample Integrity.
- How is the muffle furnace packaged for shipping? Ensuring Safe Delivery for Your Lab Equipment
- What is the specific role of a Muffle Furnace in processing solar cell coatings? Unlock Superior Durability and Efficiency
- What types of analyses can be performed using a muffle furnace in coal analysis? Unlock Key Coal Quality Insights



















