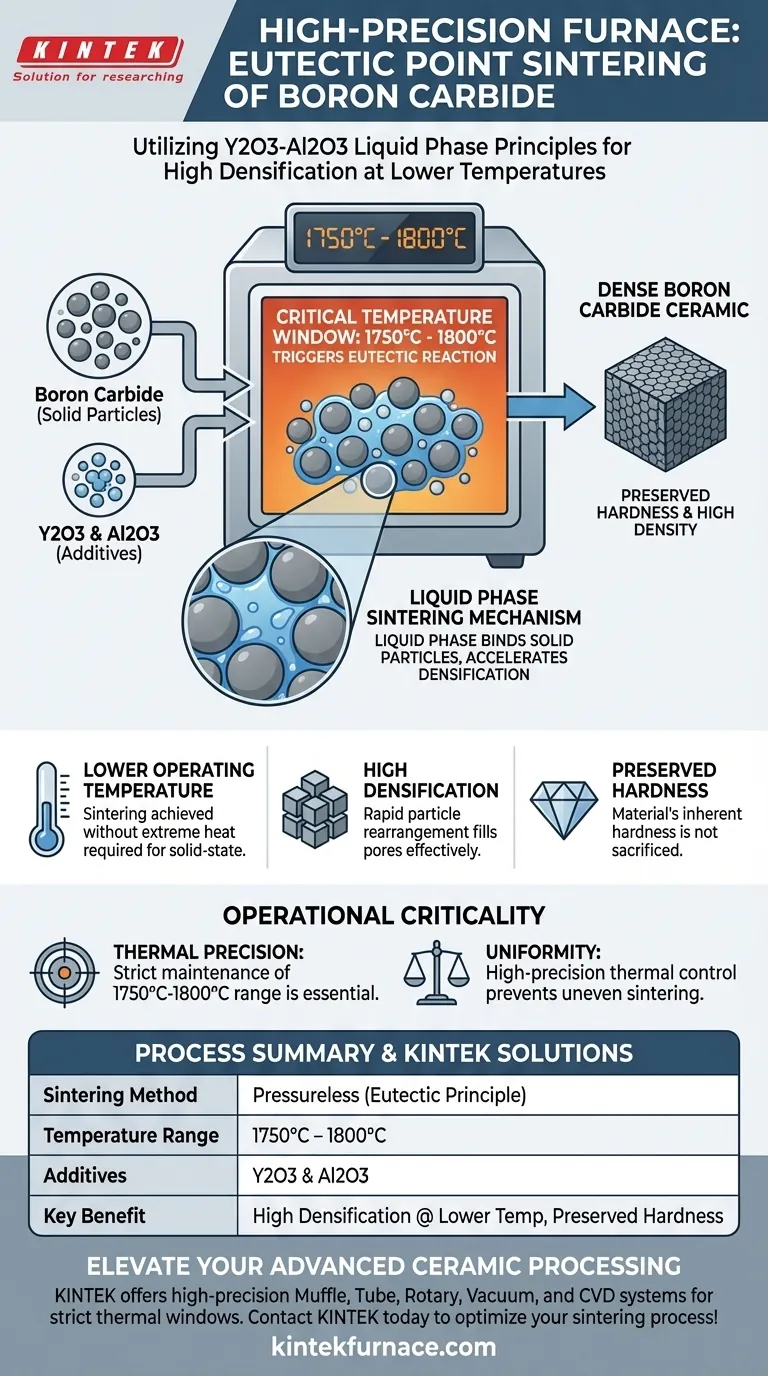
A high-precision high-temperature laboratory furnace utilizes eutectic point principles by strictly maintaining a thermal environment between 1750°C and 1800°C to facilitate liquid phase sintering. By holding this specific temperature window, the furnace triggers a chemical reaction in Y2O3-Al2O3 additives, turning them into a liquid phase that binds the solid Boron Carbide particles together.
The core advantage of this method is the ability to achieve high densification without extreme heat or pressure. By triggering a specific eutectic reaction, the furnace enables Boron Carbide to be sintered at lower temperatures while preserving the material's inherent hardness.

The Mechanics of Liquid Phase Sintering
To understand how the furnace achieves this, we must look at the interaction between the temperature control and the chemical additives.
The Role of the Y2O3-Al2O3 System
In this process, Boron Carbide is not sintered in isolation. It is mixed with a specific additive system consisting of Yttrium Oxide (Y2O3) and Aluminum Oxide (Al2O3).
These additives are chosen because they possess a specific eutectic point—the lowest temperature at which the mixture melts into a liquid.
Triggering the Reaction at 1750°C–1800°C
The furnace's primary function is to reach and maintain the critical temperature range of 1750°C to 1800°C.
Within this narrow window, the Y2O3 and Al2O3 react to form a liquid phase. This is not a gradual softening; it is a distinct phase change triggered by the thermal precision of the equipment.
Accelerating Mass Transfer
Once the liquid phase is formed, it acts as a carrier medium between the solid Boron Carbide particles.
The presence of this liquid significantly accelerates particle rearrangement. It fills the pores between the solid particles and facilitates mass transfer, allowing the ceramic to densify rapidly.
Lowering Process Requirements
Because the liquid phase acts as a "gluelike" transport mechanism, the Boron Carbide does not need to reach its own melting point (which is significantly higher) to fuse.
This results in a fully sintered, dense ceramic produced at significantly lower temperatures than would be required for solid-state sintering.
Operational Criticality and Trade-offs
While effective, relying on eutectic point principles introduces specific operational constraints that must be managed.
The Sensitivity of the Thermal Window
The success of this process relies entirely on the accuracy of the thermal field.
The window of operation is tight (1750°C to 1800°C). If the furnace drifts below this range, the eutectic reaction will not occur, and the additives will remain solid, preventing densification.
Material Purity vs. Additives
This method requires the introduction of foreign materials (Y2O3 and Al2O3) into the Boron Carbide matrix.
In many ceramic processes, additives can degrade mechanical properties. However, in this specific application, the reference notes that the material’s hardness is not sacrificed, suggesting a highly compatible grain boundary phase.
Equipment Capability
Standard furnaces may lack the uniformity required to hold this temperature range throughout the entire chamber.
Using a furnace without high-precision thermal controls risks uneven sintering, where parts of the sample react while others remain porous.
Making the Right Choice for Your Goal
When selecting a sintering strategy for Boron Carbide, consider your specific density and equipment requirements.
- If your primary focus is Maximum Density: Ensure your furnace can maintain a stable hold within the 1750°C–1800°C range to fully activate the liquid phase mechanism.
- If your primary focus is Process Efficiency: Utilize the Y2O3-Al2O3 additive system to lower the required operating temperature, reducing energy consumption and cycle time.
- If your primary focus is Material Hardness: Proceed with this liquid phase method, as it uniquely achieves densification without compromising the mechanical hardness of the final ceramic.
Precision in temperature control is the single most critical factor in leveraging eutectic principles for pressureless sintering.
Summary Table:
| Feature | Pressureless Sintering (Eutectic Method) |
|---|---|
| Temperature Range | 1750°C – 1800°C |
| Chemical Additives | Yttrium Oxide (Y2O3) & Aluminum Oxide (Al2O3) |
| Phase Mechanism | Liquid Phase Sintering |
| Key Benefit | High densification at lower temperatures |
| Critical Factor | Thermal uniformity & precision control |
| Final Properties | Preserved material hardness and high density |
Elevate Your Advanced Ceramic Processing
Precision temperature control is the difference between a porous sample and a perfectly densified ceramic. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the strict thermal windows required for eutectic reactions and liquid phase sintering.
Whether you are sintering Boron Carbide or developing new composite materials, our customizable lab high-temperature furnaces provide the uniformity and reliability your research demands.
Ready to optimize your sintering process? Contact KINTEK today to discuss your unique needs!
Visual Guide
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How do stirring equipment and temperature-controlled heating stages influence magnetic nanoparticle quality?
- What is the primary function of a high-temperature sintering furnace operating at 1173 K in the preparation of porous oxide precursors? Achieve Structural Integrity for Your Precursors
- What role does a high-temperature annealing furnace play in the preparation of AAO substrates? Enhance Pore Regularity
- How does an annealing furnace work? A Guide to Controlled Heat Treatment
- How does the use of a stainless steel high-pressure autoclave affect ZnS/CeO2@CNT formation? Optimize Catalyst Growth
- How does high-purity argon gas affect the production of ultrafine magnesium powder in evaporation-condensation methods? Master Particle Size Control
- What are the two methods of temperature control of resistance furnace? Optimize for Precision or Cost
- What material is used in porcelain fused to metal restoration? A Guide to Alloys & Aesthetics



















