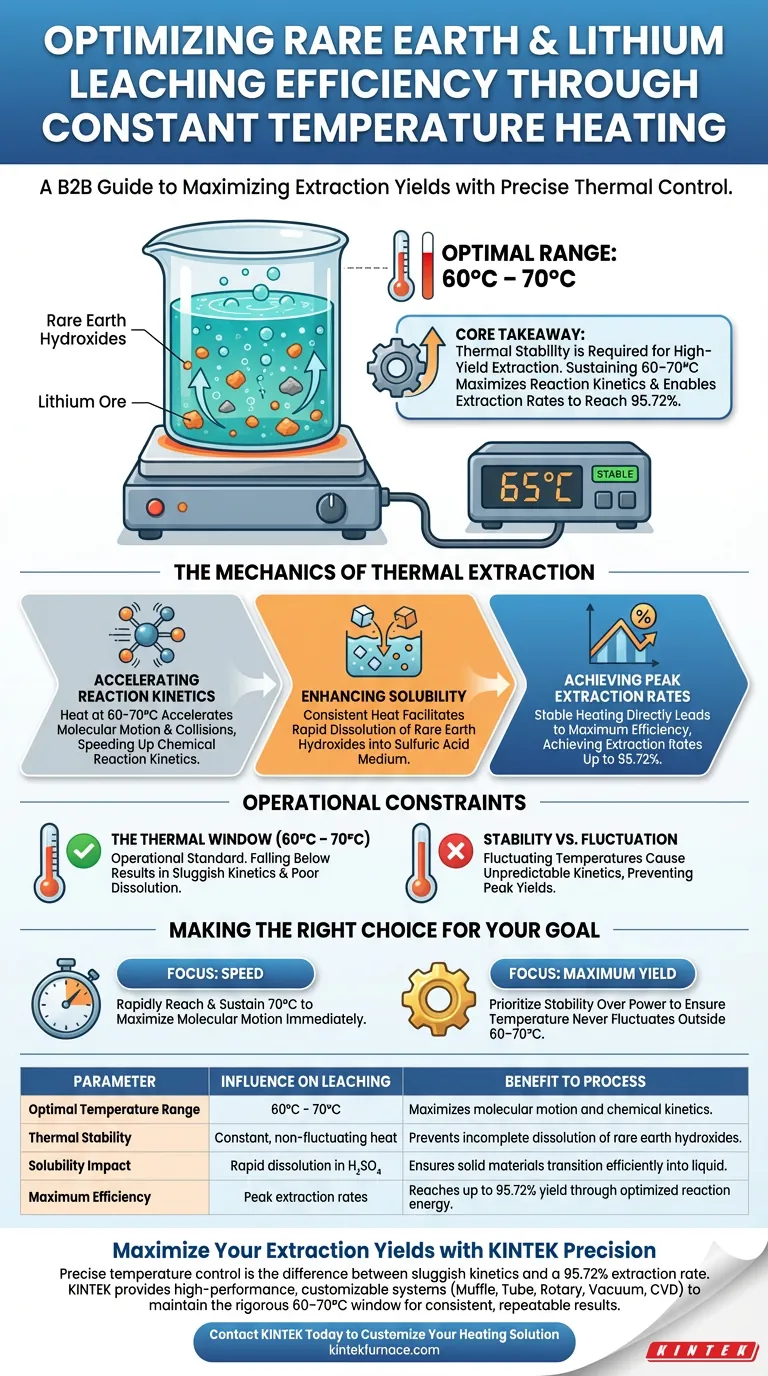
A constant temperature heating device acts as the primary driver for reaction kinetics in leaching experiments. By maintaining a precise thermal environment, specifically between 60°C and 70°C, the device accelerates molecular motion. This facilitates the rapid dissolution of rare earth hydroxides into the sulfuric acid medium, directly enhancing the leaching process.
Core Takeaway Thermal stability is not just a variable; it is a requirement for high-yield extraction. By sustaining a constant temperature within the optimal 60–70°C window, you maximize chemical reaction kinetics, enabling rare earth extraction rates to reach 95.72%.

The Mechanics of Thermal Extraction
Accelerating Reaction Kinetics
The primary function of the heating device is to provide energy to the system. Maintaining the temperature between 60°C and 70°C significantly accelerates molecular motion.
This increased motion leads to more frequent and effective collisions between the solvent and the solid material. Consequently, the chemical reaction kinetics are sped up, reducing the time required for the leaching process.
Enhancing Solubility
The stability provided by the heating device is critical for dissolving solids. Specifically, it enables rare earth hydroxides to dissolve more rapidly into the sulfuric acid medium.
Without this consistent thermal input, the solubility of these hydroxides would decrease, leading to incomplete extraction and lower overall efficiency.
Achieving Peak Extraction Rates
The ultimate measure of the device's influence is the final yield. The reference data indicates that a stable heating process is directly responsible for high performance.
When the thermal environment is correctly maintained, extraction rates for rare earths can peak at 95.72%.
Understanding Operational Constraints
The Necessity of the Thermal Window
While heat is beneficial, the specific range of 60°C to 70°C is identified as the operational standard for this process.
Falling below this range will likely result in sluggish kinetics and poor dissolution of hydroxides. The device's value lies entirely in its ability to hold this specific window without fluctuation.
Stability vs. Fluctuation
The device is described explicitly as a "constant temperature" unit.
If the equipment fails to maintain stability—allowing temperatures to drift—the reaction kinetics becomes unpredictable. This inconsistency would prevent the experiment from reaching the cited 95.72% extraction capability.
Making the Right Choice for Your Goal
To replicate the high efficiency described, you must match your equipment settings to the chemical requirements of the ore.
- If your primary focus is Speed: Ensure your device can rapidly reach and sustain the upper end of the window (70°C) to maximize molecular motion immediately.
- If your primary focus is Maximum Yield: Prioritize the stability of the device over raw power to ensure the temperature never fluctuates outside the optimal 60–70°C range, securing the 95.72% extraction rate.
Success in leaching relies less on the presence of heat and more on the precision and consistency of the thermal environment you create.
Summary Table:
| Parameter | Influence on Leaching | Benefit to Process |
|---|---|---|
| Optimal Temperature Range | 60°C - 70°C | Maximizes molecular motion and chemical kinetics. |
| Thermal Stability | Constant, non-fluctuating heat | Prevents incomplete dissolution of rare earth hydroxides. |
| Solubility Impact | Rapid dissolution in H2SO4 | Ensures solid materials transition efficiently into the liquid medium. |
| Maximum Efficiency | Peak extraction rates | Reaches up to 95.72% yield through optimized reaction energy. |
Maximize Your Extraction Yields with KINTEK Precision
Precise temperature control is the difference between sluggish kinetics and a 95.72% extraction rate. At KINTEK, we understand that your rare earth and lithium leaching experiments demand absolute thermal stability.
Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all of which are fully customizable to maintain the rigorous 60–70°C window required for your specific ore chemistry. Whether you are optimizing for speed or maximum yield, KINTEK lab high-temp furnaces offer the reliability needed to ensure consistent, repeatable results.
Ready to elevate your lab's efficiency?
Contact KINTEK Today to Customize Your Heating Solution
Visual Guide
References
- Xinglan Li, Jiangfeng Guo. Recovery of rare earths and lithium from rare earth molten salt electrolytic slag by lime transformation, co-leaching and stepwise precipitation. DOI: 10.37190/ppmp/186333
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is Physical Vapor Deposition (PVD)? Master Thin Film Coating for Enhanced Materials
- How does a heated substrate platform mitigate the coffee ring effect? Enhance Ag2Se Printing Precision
- What is the purpose of analyzing dust from furnace walls using XRD? Confirm Magnesium Evaporation in AM60 Alloy
- How do industrial molds and 10 MPa pressure impact PEEK quality? Unlock Superior Density & Structural Integrity
- How does a laboratory furnace affect chemical bonding in hybrid composites? Unlock Superior Material Strength
- How does calcination temperature affect CuO grain growth? Optimize Nanoporous Film Morphology and Crystallinity
- How does rapid water cooling equipment contribute to the stability of the foaming agent in aluminum foam precursors?
- What type of furnace was chosen for annealing silicon-based materials and what were the key requirements? Discover the Ideal Solution for Precise Heat Treatment



















