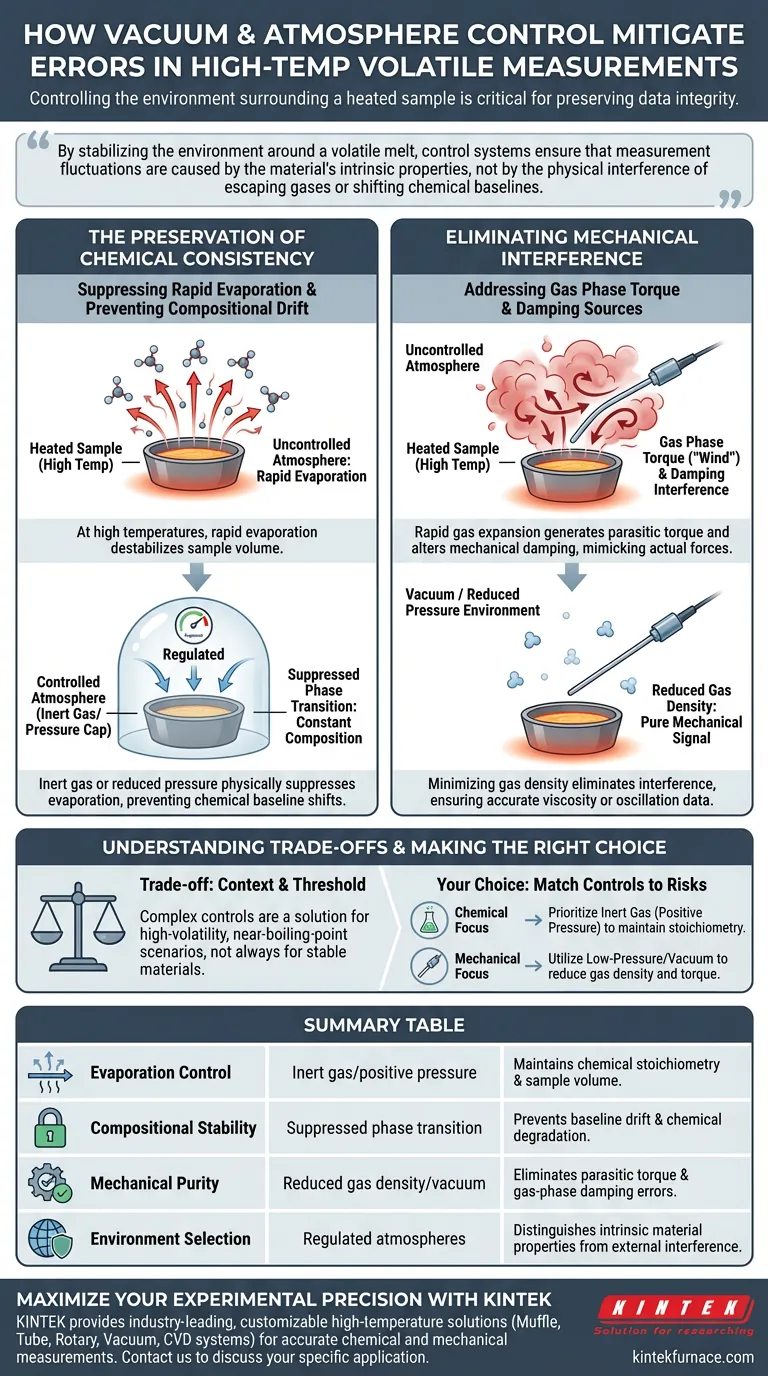
Controlling the environment surrounding a heated sample is critical for preserving data integrity. Vacuum and atmosphere control systems mitigate experimental errors by maintaining a regulated atmosphere of inert gas or low pressure, which actively suppresses the rapid evaporation of volatile components. This prevents the escaping gas phase from altering the sample's chemical makeup or generating parasitic torque that interferes with the measurement system's mechanical damping.
By stabilizing the environment around a volatile melt, control systems ensure that measurement fluctuations are caused by the material's intrinsic properties, not by the physical interference of escaping gases or shifting chemical baselines.
The Preservation of Chemical Consistency
Suppressing Rapid Evaporation
At high temperatures, volatile materials are prone to rapid evaporation, which can destabilize the sample volume. Atmosphere control systems introduce an inert gas or reduced pressure environment to physically suppress this phase transition.
Preventing Compositional Drift
When volatiles escape a melt, the chemical stoichiometry of the remaining material changes. This effectively means you are measuring a different material at the end of the experiment than at the start.
Maintaining the Baseline
By locking in the volatiles, the system ensures that the chemical composition remains constant throughout the heating cycle. This guarantees that any observed changes in physical properties are due to temperature, not chemical degradation.
Eliminating Mechanical Interference
The Problem of Gas Phase Torque
Near a material's boiling point, the rapid expansion of gas can generate physical torque on the measurement apparatus. This "wind" from the sample can mimic or mask the actual forces you are trying to measure.
Distinguishing Damping Sources
Atmosphere control systems minimize the density of the gas interacting with the sensor components. This is vital because the gas phase can interfere with the mechanical damping characteristics of the system, leading to erroneous viscosity or oscillation data.
Understanding the Trade-offs
Context is Critical
It is important to note that air damping is typically a minor factor in many standard measurements. Implementing complex vacuum or atmosphere controls is a specific solution for high-volatility scenarios, not necessarily a requirement for stable, non-volatile materials.
The Boiling Point Threshold
The utility of these systems peaks when measurements are conducted near the material's boiling point. Below this threshold, the mechanical interference of the atmosphere is often negligible, though chemical protection against oxidation may still be required.
Making the Right Choice for Your Experiment
To ensure your data accurately reflects your material's properties, align your environmental controls with your specific experimental risks.
- If your primary focus is preventing chemical changes: Prioritize an inert gas atmosphere that creates a positive pressure cap to effectively suppress evaporation and maintain stoichiometry.
- If your primary focus is mechanical signal purity: Utilize low-pressure or vacuum control to reduce gas density, ensuring that torque and damping readings are not distorted by the gas phase near the boiling point.
Control the atmosphere, and you control the reliability of your results.
Summary Table:
| Feature | Mitigation Method | Experimental Benefit |
|---|---|---|
| Evaporation Control | Inert gas/positive pressure | Maintains chemical stoichiometry and sample volume. |
| Compositional Stability | Suppressed phase transition | Prevents baseline drift and chemical degradation. |
| Mechanical Purity | Reduced gas density/vacuum | Eliminates parasitic torque and gas-phase damping errors. |
| Environment Selection | Regulated atmospheres | Distinguishes intrinsic material properties from external interference. |
Maximize Your Experimental Precision with KINTEK
Don’t let volatile evaporation or atmospheric interference compromise your research results. KINTEK provides industry-leading high-temperature solutions backed by expert R&D and precision manufacturing.
Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our laboratory furnaces are fully customizable to meet your unique atmospheric and thermal requirements. Our systems are engineered to provide the stable, regulated environments necessary for accurate chemical and mechanical measurements.
Ready to elevate your lab's performance? Contact KINTEK today to discuss your specific application and find the perfect controlled-atmosphere solution.
Visual Guide
References
- V. M. B. Nunes, C. A. Nieto de Castro. Correct Use of Oscillating-Cup Viscometers for High-Temperature Absolute Measurements of Newtonian Melts. DOI: 10.1007/s10765-024-03355-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the function of a tube atmosphere furnace? Precision Mn1/CeO2 Catalyst Reduction & Fabrication
- What are the considerations for air atmosphere and cooling in Inconel 625 heat treatment? Optimize 3D Part Stability
- Why is a high-precision furnace essential for CZTSSe thin films? Prevent Phase Decomposition and Amorphization
- Why are retort furnaces significant in industrial applications? Unlock Precision Heat Treatment and Superior Material Quality
- What is the primary purpose of using a small controlled electric furnace? Optimize Black Liquor Pyrolysis for Research
- What is the core difference between box and atmosphere furnaces? Choose the Right Equipment for Your Lab
- What makes inert atmosphere furnaces different from standard tube furnaces? Key Benefits for Material Protection
- What is the role of a reducing atmosphere in foundry operations? Prevent Oxidation and Control Metal Quality



















