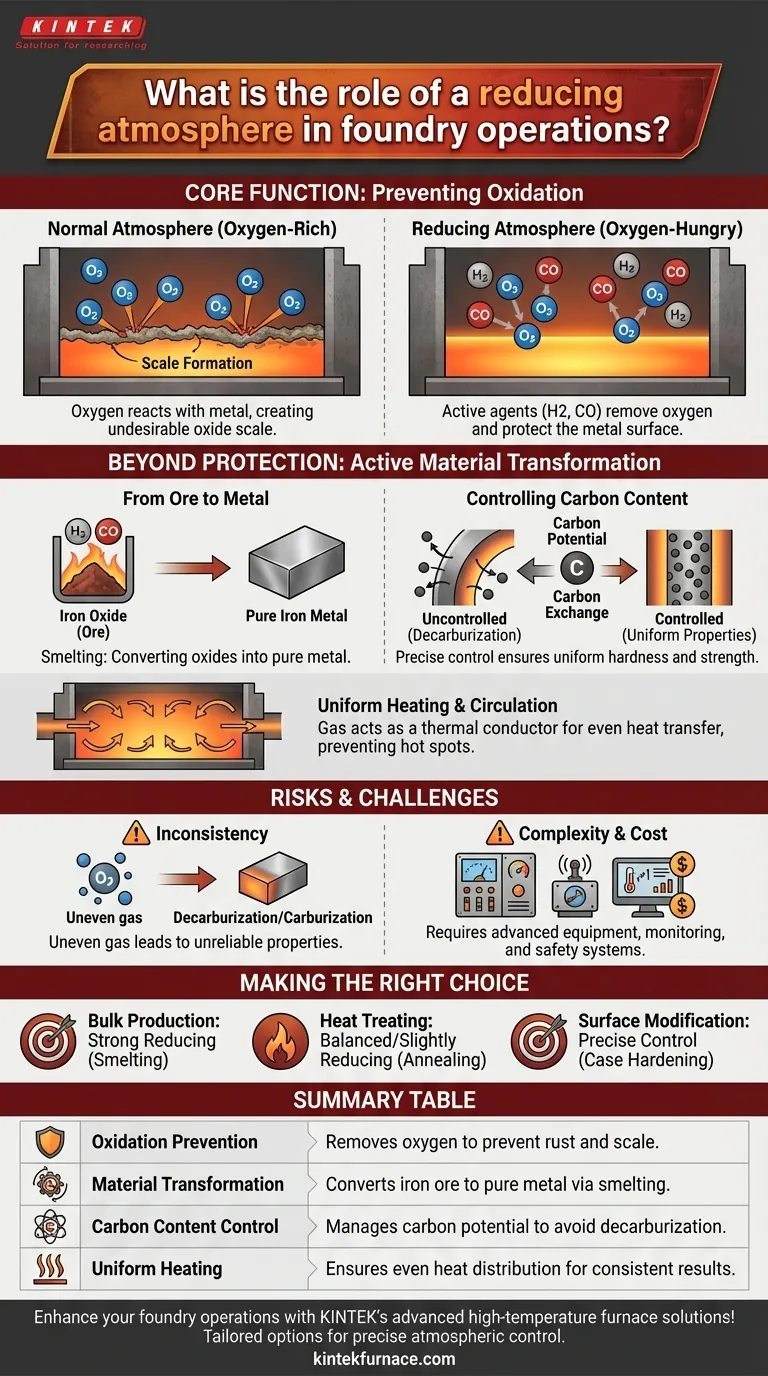
At its core, the role of a reducing atmosphere in foundry operations is to chemically control the environment during high-temperature processes. It actively prevents unwanted reactions like oxidation (rust and scale) and can be used to intentionally transform materials, such as converting purified iron ore into pure iron metal.
The critical takeaway is that a reducing atmosphere is not merely a passive shield. It is an active chemical agent that removes oxygen, allowing operators to protect the metal's surface, control its chemical composition, and ensure the final product meets precise metallurgical specifications.
The Core Function: Preventing Oxidation
What Happens in a Normal Atmosphere?
When metal is heated to high temperatures in the presence of normal air, the oxygen aggressively reacts with the metal's surface. This process is called oxidation.
The result is the formation of a layer of oxide, commonly known as scale. This scale is undesirable as it damages the surface finish, alters the part's dimensions, and can flake off, creating defects.
How a Reducing Atmosphere Fights Back
A reducing atmosphere is engineered to be "oxygen-hungry." It is primarily composed of gases like hydrogen (H₂) and carbon monoxide (CO).
These gases have a stronger affinity for oxygen than the metal does at high temperatures. They actively seek out and combine with any free oxygen in the furnace, and can even strip oxygen atoms directly from oxides already on the metal's surface, effectively cleaning it.
Beyond Protection: Active Material Transformation
While preventing oxidation is a primary role, a reducing atmosphere is also a powerful tool for intentionally changing the material itself.
From Ore to Metal
In the most fundamental foundry process, a reducing atmosphere is used to smelt ore. Purified iron ore is essentially iron oxide.
By heating this ore in a potent reducing atmosphere, the carbon monoxide and hydrogen strip the oxygen away from the iron oxide, leaving behind pure, molten iron.
Controlling Carbon Content
The atmosphere's composition also dictates the carbon exchange between the furnace environment and the metal part, particularly with steel. This is known as carbon potential.
An uncontrolled atmosphere can lead to decarburization, where carbon is stripped from the surface of the steel. This makes the surface softer and weaker than the core, which is often a critical failure.
A precisely controlled reducing atmosphere prevents this loss, ensuring uniform hardness and strength throughout the component.
A Medium for Uniform Heating
Finally, the gas in an atmosphere furnace is not static. It circulates and acts as a thermal conductor.
This ensures heat is transferred evenly from the furnace elements to all surfaces of the part, preventing hot spots and ensuring a uniform and predictable heat treatment.
Understanding the Risks and Challenges
Using a reducing atmosphere provides immense control, but it also introduces complexity and potential pitfalls that must be managed.
The Danger of Inconsistency
An improperly mixed or circulated atmosphere can be more harmful than using no protective atmosphere at all.
If the concentration of reducing gases is not uniform, some areas of a part can experience decarburization while others might see unintended carburization (the addition of carbon). This creates inconsistent and unreliable material properties.
Process Complexity and Cost
Atmosphere furnaces are inherently more complex and costly to operate than simple air furnaces.
They require sophisticated gas mixing panels, monitoring equipment (like oxygen probes and dew point sensors), and robust safety systems, as hydrogen and carbon monoxide are flammable and toxic.
Making the Right Choice for Your Goal
The specific goal of your furnace operation dictates the type and control level of the atmosphere required.
- If your primary focus is bulk metal production from ore: A strong, highly reducing atmosphere is essential for efficiently converting oxides into pure metal.
- If your primary focus is heat treating parts (e.g., annealing): The goal is a balanced or slightly reducing atmosphere to simply prevent surface damage like oxidation and decarburization during the thermal cycle.
- If your primary focus is modifying surface properties (e.g., case hardening): The atmosphere must be precisely controlled to manage carbon potential, actively adding a controlled amount of carbon to the component's surface.
Ultimately, mastering the furnace atmosphere is the key to achieving precise and repeatable control over the final quality of any high-performance metal component.
Summary Table:
| Aspect | Role of Reducing Atmosphere |
|---|---|
| Oxidation Prevention | Removes oxygen to prevent rust and scale formation |
| Material Transformation | Converts iron ore to pure metal via smelting |
| Carbon Content Control | Manages carbon potential to avoid decarburization or carburization |
| Uniform Heating | Ensures even heat distribution for consistent results |
Enhance your foundry operations with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored options like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise control over reducing atmospheres to meet your unique experimental needs—prevent oxidation, achieve uniform heating, and optimize material transformations. Contact us today to discuss how our expertise can improve your metal processing quality and efficiency!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality



















