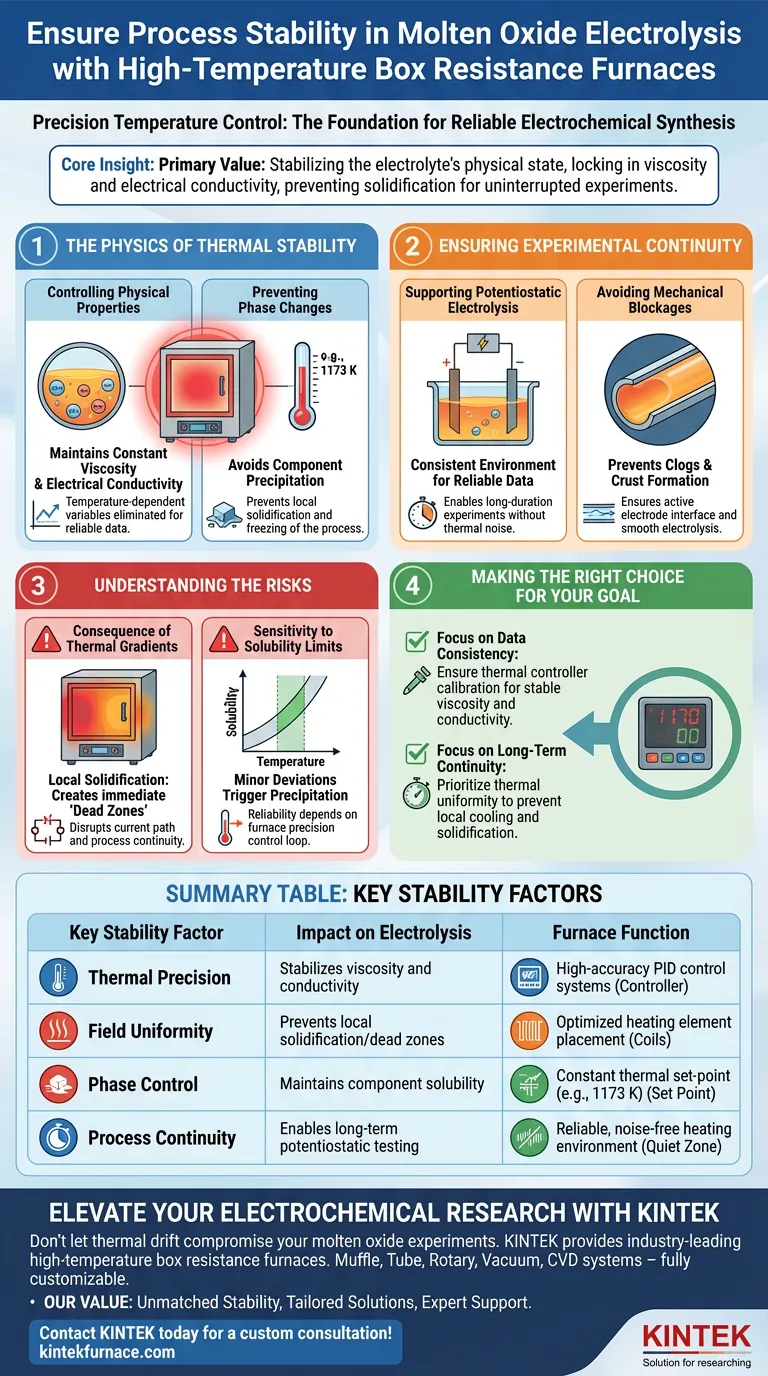
High-temperature box resistance furnaces ensure process stability by utilizing precision temperature control systems to maintain a rigid thermal set point, such as 1173 K. This precise regulation creates a constant thermal field, which is the foundational requirement for successful molten oxide electrolysis.
Core Insight: The primary value of these furnaces is not just generating heat, but stabilizing the electrolyte's physical state. By keeping temperature constant, the furnace locks in the viscosity and electrical conductivity of the melt, preventing solidification and ensuring the experiment runs without interruption.

The Physics of Thermal Stability
Controlling Physical Properties
The success of electrolysis depends heavily on the physical state of the electrolyte. Viscosity and electrical conductivity are temperature-dependent variables.
By maintaining a stable thermal field, the furnace ensures these properties remain constant throughout the experiment. This eliminates variables that could otherwise skew data or alter the electrochemical behavior of the melt.
Preventing Phase Changes
Temperature fluctuations can have catastrophic effects on the solubility of components within the molten oxide.
If the temperature drops locally, the solubility of certain components may decrease. This leads to the precipitation of components or local solidification of the electrolyte, effectively freezing the process in specific areas.
Ensuring Experimental Continuity
Supporting Potentiostatic Electrolysis
Potentiostatic electrolysis requires a consistent environment to yield reliable data.
The stability provided by the box resistance furnace ensures that the applied potential drives the reaction under uniform conditions. This consistency allows for long-duration experiments without the noise caused by thermal drift.
Avoiding Mechanical Blockages
The continuity of the experiment is directly linked to the fluidity of the electrolyte.
By preventing the precipitation of solids due to solubility changes, the system avoids clogs or crust formation. This ensures the electrode interface remains active and the electrolysis proceeds smoothly.
Understanding the Risks
The Consequence of Thermal Gradients
While these furnaces are designed for stability, any failure in the control system can be detrimental.
If the thermal field becomes uneven, local solidification creates immediate "dead zones" in the electrolyte. This disrupts the current path and ruins the continuity of the process.
Sensitivity to Solubility Limits
The process relies heavily on operating within a specific solubility window.
Even minor deviations from the target temperature (e.g., 1173 K) can trigger unwanted precipitation. Therefore, the reliability of the experiment is entirely dependent on the precision of the furnace's control loop.
Making the Right Choice for Your Goal
If your primary focus is data consistency: Ensure your furnace's thermal controller is calibrated to maintain viscosity and conductivity, as these directly impact electrochemical measurements.
If your primary focus is long-term operational continuity: Prioritize thermal uniformity to prevent local cooling, which triggers solidification and precipitation that can halt the experiment.
Precision temperature control is the invisible force that transforms a chaotic molten environment into a stable platform for electrochemical synthesis.
Summary Table:
| Key Stability Factor | Impact on Electrolysis | Furnace Function |
|---|---|---|
| Thermal Precision | Stabilizes viscosity and conductivity | High-accuracy PID control systems |
| Field Uniformity | Prevents local solidification/dead zones | Optimized heating element placement |
| Phase Control | Maintains component solubility | Constant thermal set-point (e.g., 1173 K) |
| Process Continuity | Enables long-term potentiostatic testing | Reliable, noise-free heating environment |
Elevate Your Electrochemical Research with KINTEK
Don't let thermal drift compromise your molten oxide experiments. KINTEK provides industry-leading high-temperature box resistance furnaces engineered for the rigorous demands of material science. Backed by expert R&D and precision manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your specific research parameters.
Our value to you:
- Unmatched Stability: Ensure constant viscosity and electrical conductivity in your melts.
- Tailored Solutions: Custom furnace configurations designed for your unique electrochemical setups.
- Expert Support: Technical guidance to help you eliminate thermal gradients and local solidification.
Ready to ensure the success of your next experiment? Contact KINTEK today for a custom consultation!
Visual Guide
References
- Joongseok Kim, Kyung‐Woo Yi. Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. DOI: 10.3390/ma18174116
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a muffle furnace play in silver film transformation? Master Nanoparticle Self-Assembly with Precision
- What is the primary purpose of using a muffle furnace for MAL calcination? Unlock the Structure Memory Effect
- What are the construction features and temperature capabilities of a muffle furnace? Key Insights for Your Lab
- What is the purpose of using a high-temperature oven for the pretreatment of anhydrous calcium chloride?
- What roles do programmable laboratory high-temperature furnaces play in calcium perrhenate single crystal preparation?
- How does a muffle furnace operate? Uncover Its Efficient, Contamination-Free Heating Process
- Why is the separation of chambers important in a muffle furnace? Ensure Purity and Uniform Heating
- What is the role of a high-temperature muffle furnace in the annealing process of para-aramid fibers?



















