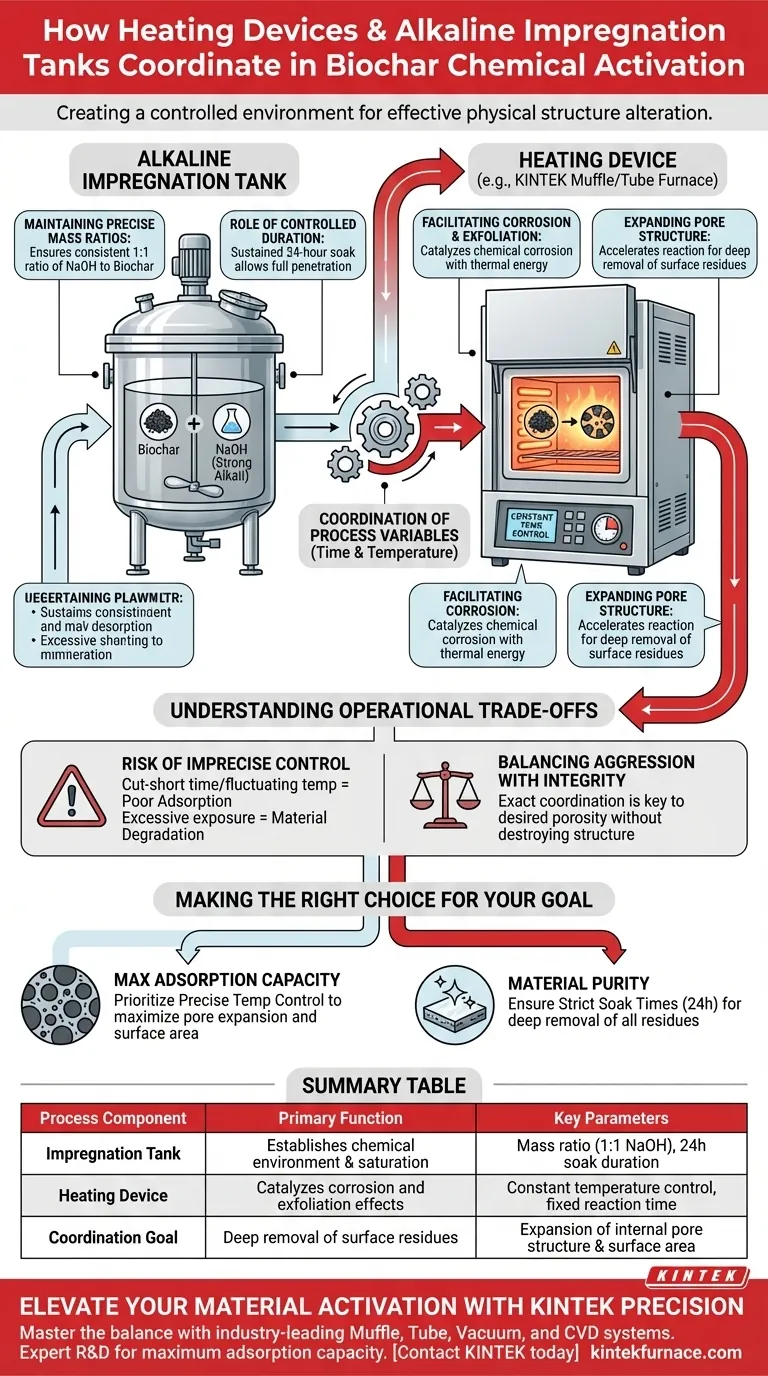
The coordination between heating devices and alkaline impregnation tanks centers on creating a controlled environment where chemical agents can effectively alter the physical structure of biochar. While the impregnation tanks maintain precise mass ratios of strong alkalis (such as NaOH) to biochar, the heating devices regulate the temperature over fixed durations to ensure thorough activation.
The success of this process relies on the strict control of reaction variables to trigger chemical corrosion and exfoliation. By managing time and temperature, these devices facilitate the deep removal of surface residues and the expansion of the pore structure, directly determining the material's final adsorption performance.

The Mechanics of Chemical Activation
Maintaining Precise Mass Ratios
The primary function of the impregnation tank is to establish and hold a specific chemical environment.
It ensures a consistent mass ratio between the activating agent and the raw material, such as a 1:1 ratio of NaOH to biochar. This balance is critical to ensure there is enough alkali to coat the material without wasting resources.
The Role of Controlled Duration
Chemical activation is not instantaneous; it requires a sustained soak to be effective.
The tanks are designed to hold the mixture for a set duration, often lasting 24 hours. This allows the strong alkalis to fully penetrate the biochar matrix before the heating phase drives the reaction further.
Driving Structure Change Through Heat
Facilitating Corrosion and Exfoliation
Once the mixture is prepared, the heating device acts as the catalyst for the physical transformation of the material.
By raising and maintaining the temperature, the device activates the chemical corrosion and exfoliation effects of the strong alkalis. This thermal energy allows the NaOH to aggressively attack surface impurities that would otherwise block the biochar's potential.
Expanding the Pore Structure
The ultimate goal of this coordination is the modification of the biochar's internal architecture.
Heat accelerates the reaction, leading to the deep removal of surface residues. This process clears the way for a significant expansion of the biochar pore structure, which is the primary factor in improving adsorption performance.
Understanding the Operational Trade-offs
The Risk of Imprecise Control
Because this process relies on "deep removal" and "exfoliation," precision is paramount.
If the reaction time is cut short or the temperature fluctuates, surface residues may remain, resulting in biochar with poor adsorption capabilities. Conversely, excessive exposure could degrade the material beyond utility.
Balancing Aggression with Integrity
The process utilizes strong alkalis, which are inherently destructive agents.
The coordination between the tank and heating device must be exact to achieve the desired porosity without destroying the structural integrity of the biochar itself.
Making the Right Choice for Your Goal
To maximize the utility of your biochar, you must tune the coordination of these devices to your specific performance metrics.
- If your primary focus is maximum adsorption capacity: Prioritize precise temperature control to maximize the expansion of the pore structure and surface area.
- If your primary focus is material purity: Ensure strictly maintained soak times (e.g., 24 hours) to guarantee the deep removal of all surface residues.
Mastering the variable interplay between heat and chemical saturation is the only way to transform raw biochar into a high-performance adsorbent.
Summary Table:
| Process Component | Primary Function | Key Parameters |
|---|---|---|
| Impregnation Tank | Establishes chemical environment & saturation | Mass ratio (e.g., 1:1 NaOH), 24h soak duration |
| Heating Device | Catalyzes corrosion and exfoliation effects | Constant temperature control, fixed reaction time |
| Coordination Goal | Deep removal of surface residues | Expansion of internal pore structure & surface area |
Elevate Your Material Activation with KINTEK Precision
To achieve superior biochar performance, you need equipment that masters the delicate balance of heat and chemical saturation. KINTEK provides industry-leading Muffle, Tube, Vacuum, and CVD systems, backed by expert R&D to ensure your activation process reaches maximum adsorption capacity.
Whether you require precise temperature regulation for pore expansion or customizable lab high-temp furnaces for unique chemical workflows, KINTEK is your trusted partner in manufacturing excellence.
Ready to optimize your lab's efficiency? Contact KINTEK today to discuss our customizable furnace solutions.
Visual Guide
References
- Barbara Pieczykolan. Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge. DOI: 10.3390/ijms26167912
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of ZrCp(NMe2)3? Master Precision Surface Passivation in Area-Selective ALD
- What is the significance of temperature control precision in high-temperature furnaces for carbon-doped titanium dioxide?
- Why is annealing in a heat treatment furnace performed on graphite flake/copper composite samples before performance testing? Ensure Data Integrity for Precision Thermal Expansion Measurements
- What is the role of carbonaceous reducing agents in copper slag treatment? Maximize Metal Recovery with Expert Insights
- How do stirring equipment and temperature-controlled heating stages influence magnetic nanoparticle quality?
- How is the thermal stability of KBaBi compounds evaluated? Discover Precise XRD & Heat Treatment Limits
- What is the purpose of preheating reinforcement particles? Optimize AMC Stir Casting Results
- What is the basic principle of a sintering furnace? Transform Powder into Dense, Strong Components



















